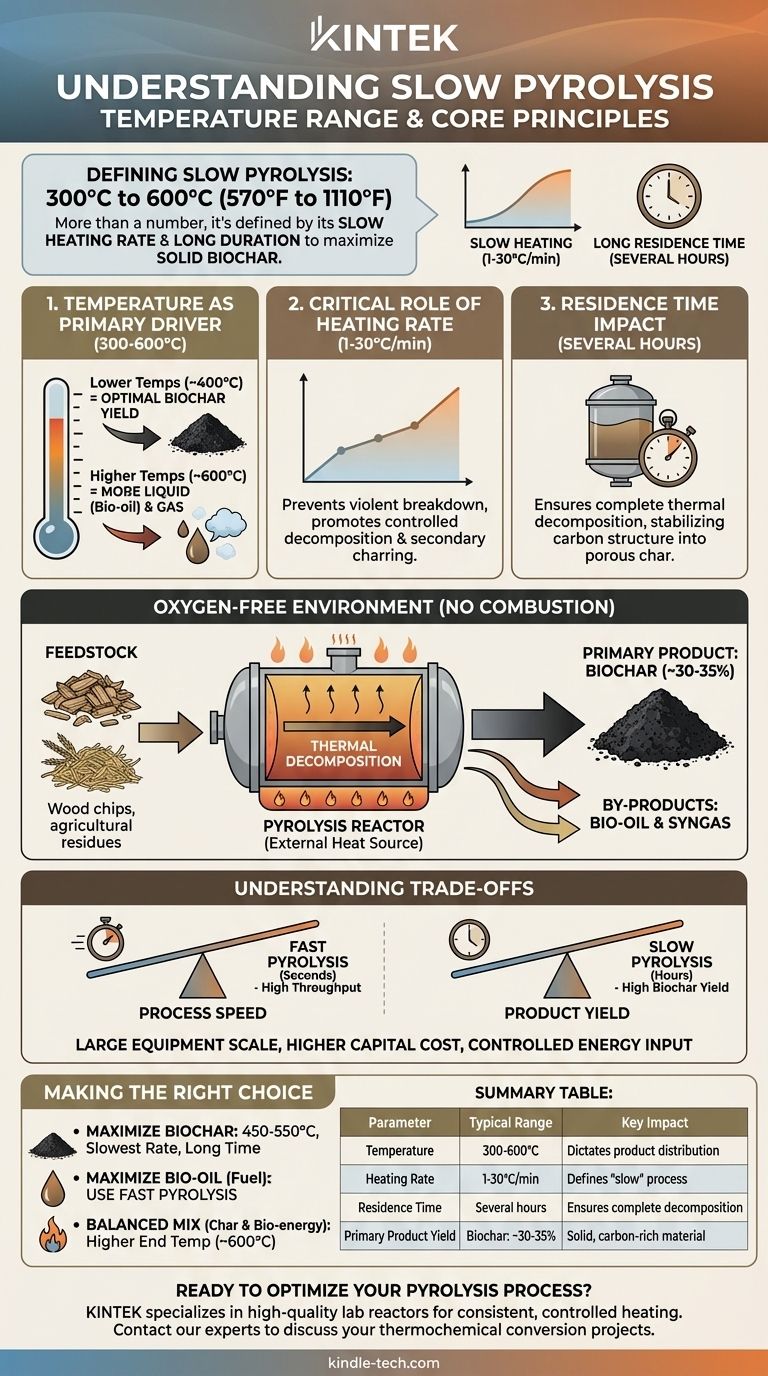
To define slow pyrolysis, you must look beyond a single number. While the process typically operates in a temperature range of 300°C to 600°C (570°F to 1110°F), its defining characteristic is actually the slow heating rate and long duration, which are deliberately controlled to maximize the production of solid biochar.
The critical insight is that slow pyrolysis isn't just about reaching a certain temperature, but about how slowly you get there. This gradual, controlled heating process prioritizes the formation of a stable, carbon-rich solid (biochar) over the liquids and gases favored by faster methods.

The Core Principles of Slow Pyrolysis
Slow pyrolysis is a thermochemical conversion process engineered for a specific outcome. Understanding its three core parameters—temperature, heating rate, and residence time—is key to controlling the final product.
Temperature as the Primary Driver
The target temperature dictates which chemical bonds within the biomass break down. Operating between 300°C and 600°C is the typical range for slow pyrolysis.
Temperatures at the lower end of this range (around 400°C) are optimal for maximizing the yield of solid biochar. As temperatures climb toward 600°C and beyond, secondary reactions begin to crack larger molecules, increasing the yield of liquid (bio-oil) and gas products at the expense of the char.
The Critical Role of Heating Rate
The heating rate is what truly makes the process "slow." It is typically maintained between 1°C and 30°C per minute.
This gradual increase in temperature prevents the violent and rapid breakdown of organic matter. Instead, it allows for controlled, sequential decomposition and secondary reactions that polymerize volatile compounds back onto the solid surface, further increasing biochar yield.
Residence Time and Its Impact
Slow pyrolysis involves very long residence times, often lasting several hours. This is the total time the biomass spends inside the reactor at the target temperature.
This extended duration ensures that the thermal decomposition is complete, driving off remaining volatile materials and allowing the carbon structure to stabilize and form a high-quality, porous char. This stands in stark contrast to fast pyrolysis, where residence times are measured in seconds.
The Oxygen-Free Environment
Crucially, pyrolysis of any kind must occur in an environment with very limited or zero oxygen. This ensures the material thermally decomposes rather than combusts (burns). The energy required to heat the reactor is supplied externally, sometimes by burning a portion of the gas produced by the process itself.
How Process Conditions Dictate Product Yields
The goal of slow pyrolysis is almost always to maximize one product: biochar. The other outputs, bio-oil and syngas, are considered by-products, though they have value.
Maximizing Biochar Yield
To produce the most biochar, you use a combination of a relatively low peak temperature (e.g., 450-550°C), a very slow heating rate, and a long residence time. This recipe promotes the secondary charring reactions that are the hallmark of the process.
The Production of Bio-oil and Syngas
While minimized, slow pyrolysis still produces some liquid (bio-oil) and non-condensable gases (syngas). These are formed from the volatile compounds that escape the biomass and are not re-polymerized onto the char. Their yield increases with higher operating temperatures.
The Influence of Feedstock
The type and condition of the starting material (feedstock) also have a major impact. Drier, denser materials like wood chips will produce different results than lighter, wetter materials like agricultural residues, even under identical process conditions.
Understanding the Trade-offs
Choosing slow pyrolysis involves a clear trade-off between process speed and the desired product characteristics.
Process Speed vs. Product Yield
The most obvious trade-off is time. Slow pyrolysis has a very low throughput compared to fast pyrolysis, which can process material in seconds. The benefit of this slow speed is a significantly higher yield of solid biochar (often 30-35% by weight, versus ~12% for fast pyrolysis).
Equipment Scale and Cost
Because the process requires material to be held at temperature for hours, reactors for slow pyrolysis (such as rotary kilns) must be large to achieve meaningful production volumes. This can lead to higher capital costs and a larger physical footprint compared to more compact, fast pyrolysis systems.
Energy Balance
The long duration of the process requires a sustained and controlled energy input. While the produced syngas can be combusted to provide some of this heat, the overall energy balance must be carefully managed to ensure the process is efficient.
Making the Right Choice for Your Goal
Controlling the parameters of slow pyrolysis allows you to engineer the output for a specific purpose.
- If your primary focus is maximizing high-quality, stable biochar for soil amendment or carbon sequestration: Operate in the 450-550°C range with the slowest possible heating rate and a long residence time.
- If your primary focus is high throughput and liquid bio-oil for fuel: Slow pyrolysis is the wrong process; you should investigate fast pyrolysis, which uses extremely high heating rates and short residence times.
- If your primary focus is producing a balanced mix of char and bio-energy: You can operate at the higher end of the slow pyrolysis temperature range (around 600°C) to increase the gas and liquid yield, which can then be used to power the system or for other applications.
Ultimately, mastering the interplay between temperature, heating rate, and time is the key to unlocking the full potential of thermochemical conversion.
Summary Table:
| Parameter | Typical Range for Slow Pyrolysis | Key Impact |
|---|---|---|
| Temperature | 300°C to 600°C (570°F to 1110°F) | Dictates product distribution; lower temps favor biochar. |
| Heating Rate | 1°C to 30°C per minute | Defines the "slow" process; maximizes biochar yield. |
| Residence Time | Several hours | Ensures complete decomposition and stable char formation. |
| Primary Product Yield | Biochar: ~30-35% | Solid, carbon-rich material for soil amendment and sequestration. |
Ready to optimize your pyrolysis process for maximum biochar yield?
The precise control of temperature, heating rate, and residence time is critical for successful slow pyrolysis. KINTEK specializes in high-quality lab reactors and pyrolysis systems that deliver the consistent, controlled heating required for reproducible results.
Whether you are researching biochar properties, developing new conversion techniques, or scaling up production, our equipment is engineered for reliability and precision.
Contact our experts today to discuss your specific needs and discover how KINTEK's laboratory solutions can advance your thermochemical conversion projects.
Visual Guide
Related Products
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical Laboratory Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- What is the range of pyrolysis? Master Temperature Control for Optimal Bio-Product Yields
- What are the main types of biomass conversion processes? Unlock the Best Pathway for Your Energy Needs
- What are the process advantages of using a rotary tube furnace for WS2 powder? Achieve Superior Material Crystallinity
- What temperature is needed for pyrolysis waste? A Guide to Optimizing Your Waste-to-Value Process
- What is the difference between pyrolysis combustion and gasification? A Guide to Thermal Conversion Technologies



















