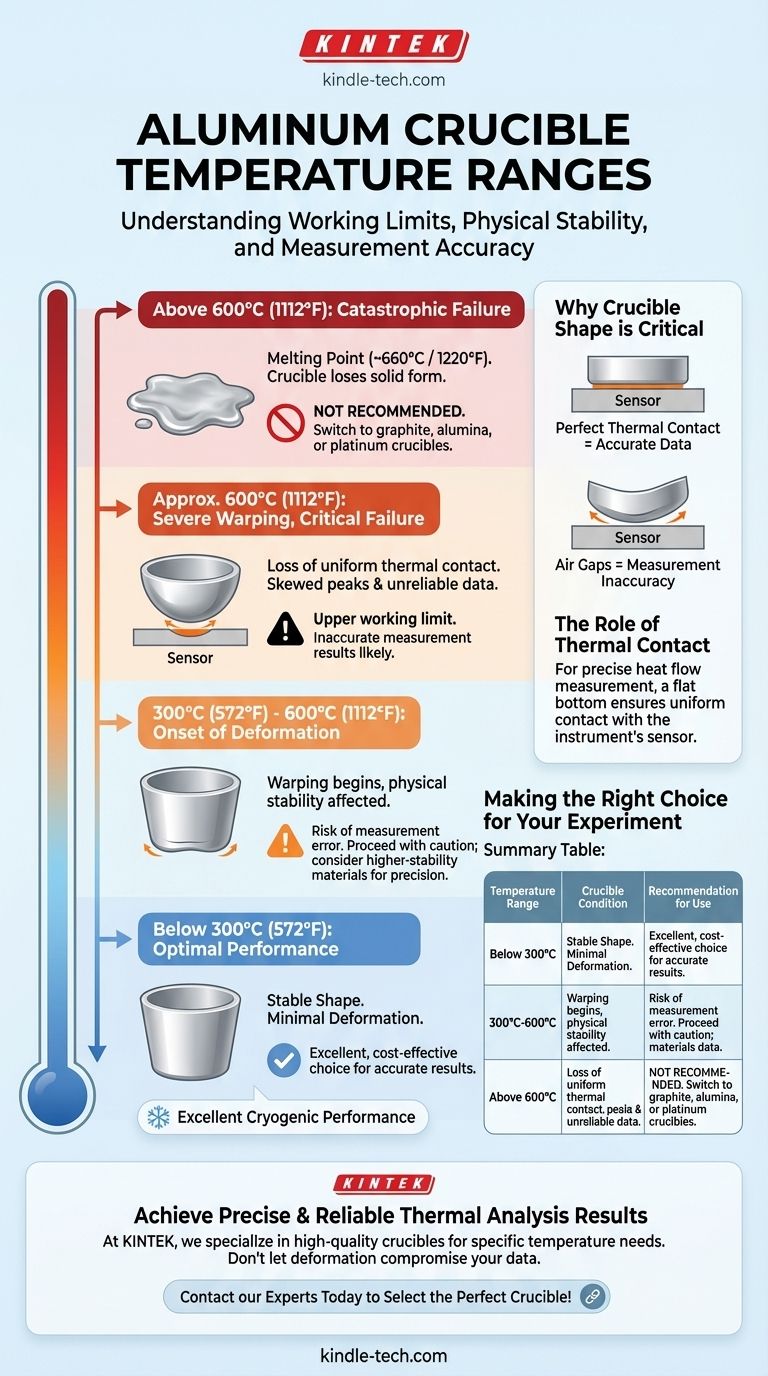
In practical terms, a standard aluminum crucible has a reliable upper working limit of approximately 600°C (1112°F). However, physical deformation that can compromise measurement accuracy begins to occur at temperatures as low as 300°C (572°F).
The critical factor isn't the melting point of aluminum (~660°C), but its physical stability. The crucible's shape begins to warp long before it melts, which can ruin the thermal contact necessary for accurate scientific measurements.

Understanding the Upper Temperature Limit
The performance of an aluminum crucible is defined by its physical integrity under heat. While it can withstand high temperatures, its usefulness for precise measurements degrades well before it fails completely.
The Onset of Deformation
Around 300°C, the flat bottom of a standard aluminum crucible can begin to deform. This initial warping is subtle but marks the beginning of the material losing its rigidity.
Severe Warping and Measurement Error
By 600°C, the deformation can become severe. The bottom of the crucible may warp so much that it only makes a single point of contact with the instrument's sensor plate.
This loss of contact is a critical failure for thermal analysis techniques like Differential Scanning Calorimetry (DSC), as it prevents uniform heat transfer and invalidates the data.
The Absolute Limit: Melting Point
The melting point of pure aluminum is approximately 660°C (1220°F). This is the temperature of catastrophic failure where the crucible will lose its solid form entirely. For any practical application, you must stay well below this temperature.
Why Crucible Shape is Critical
In thermal analysis, the physical shape of the crucible is not just a container; it is part of the measurement apparatus. Its integrity is essential for acquiring accurate data.
The Role of Thermal Contact
For an instrument to accurately measure heat flow into or out of a sample, the crucible must have a perfectly flat bottom. This ensures maximum, uniform contact with the instrument's sensor.
How Deformation Creates Inaccuracy
When the crucible bottom warps, it lifts away from the sensor, creating insulating gaps of air. This uneven thermal contact disrupts the measurement of heat flow, leading to skewed peaks, shifted transition temperatures, and ultimately, unreliable results.
Understanding the Trade-offs
While cost-effective and common, aluminum crucibles have clear limitations that dictate their proper use.
Benefit: Excellent Low-Temperature Performance
Aluminum does not become brittle at cryogenic temperatures. It maintains its strength and ductility, making it an excellent choice for experiments running well below 0°C. The lower limit is typically defined by the instrument, not the crucible.
Limitation: Pressure in Sealed Crucibles
When using hermetically sealed crucibles, pressure can build up inside from sample off-gassing. This internal pressure can accelerate and worsen the deformation of the crucible bottom as temperatures rise.
Making the Right Choice for Your Experiment
Selecting the correct crucible is fundamental to the success of your thermal analysis. Your decision should be based entirely on the temperature range of your experiment.
- If your experiment runs below 300°C: An aluminum crucible is an excellent, cost-effective choice that will provide reliable and repeatable results.
- If your experiment is between 300°C and 600°C: Proceed with caution. While usable, be aware of potential deformation that could impact precision. For high-accuracy needs, consider a more stable material.
- If your experiment will exceed 600°C: You must use a different type of crucible. Materials like graphite, alumina, or platinum are designed for these higher temperature ranges.
Matching your crucible material to your experimental needs is the first step toward achieving accurate and trustworthy data.
Summary Table:
| Temperature Range | Crucible Condition | Recommendation for Use |
|---|---|---|
| Below 300°C (572°F) | Stable, minimal deformation | Excellent, cost-effective choice |
| 300°C - 600°C (572°F - 1112°F) | Begins to warp, risk of measurement error | Use with caution; consider higher-stability materials for precision |
| Above 600°C (1112°F) | Severe deformation, potential melting | Not recommended; switch to graphite, alumina, or platinum crucibles |
Achieve precise and reliable thermal analysis results with the right crucible for your application. The integrity of your crucible is critical for accurate DSC measurements. At KINTEK, we specialize in providing high-quality lab equipment and consumables, including a full range of crucibles designed for specific temperature ranges and experimental needs. Don't let crucible deformation compromise your data. Contact our experts today to select the perfect crucible for your lab's requirements and ensure the success of your experiments. Get in touch with us now!
Visual Guide
Related Products
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
People Also Ask
- Why are High-purity Alumina Crucibles selected for corrosion testing? Ensure Data Fidelity in Molten Salt Experiments
- Why Use Alumina Crucibles for TGA of Bicyclic Carbonates? Ensure Data Purity & Chemical Inertness
- What are the advantages of selecting an alumina crucible for TGA? Ensure High-Precision Thermal Analysis Data
- What temperature can alumina crucible withstand? A Guide to High-Temperature Stability and Safety
- What is the purpose of using alumina crucibles as liners in autoclaves? Ensure Purity in High-Pressure Static Tests



















