At its core, an ashing furnace is a high-temperature oven used for a specific analytical purpose: to completely burn away the organic material from a sample. This process leaves behind only the inorganic, non-combustible residue, known as ash, which can then be collected, weighed, and analyzed.
The fundamental use of an ashing furnace is not simply to burn a sample, but to perform a precise separation. It is a critical sample preparation technique that isolates the inorganic content of a material for quantitative analysis, essential for quality control and material science.

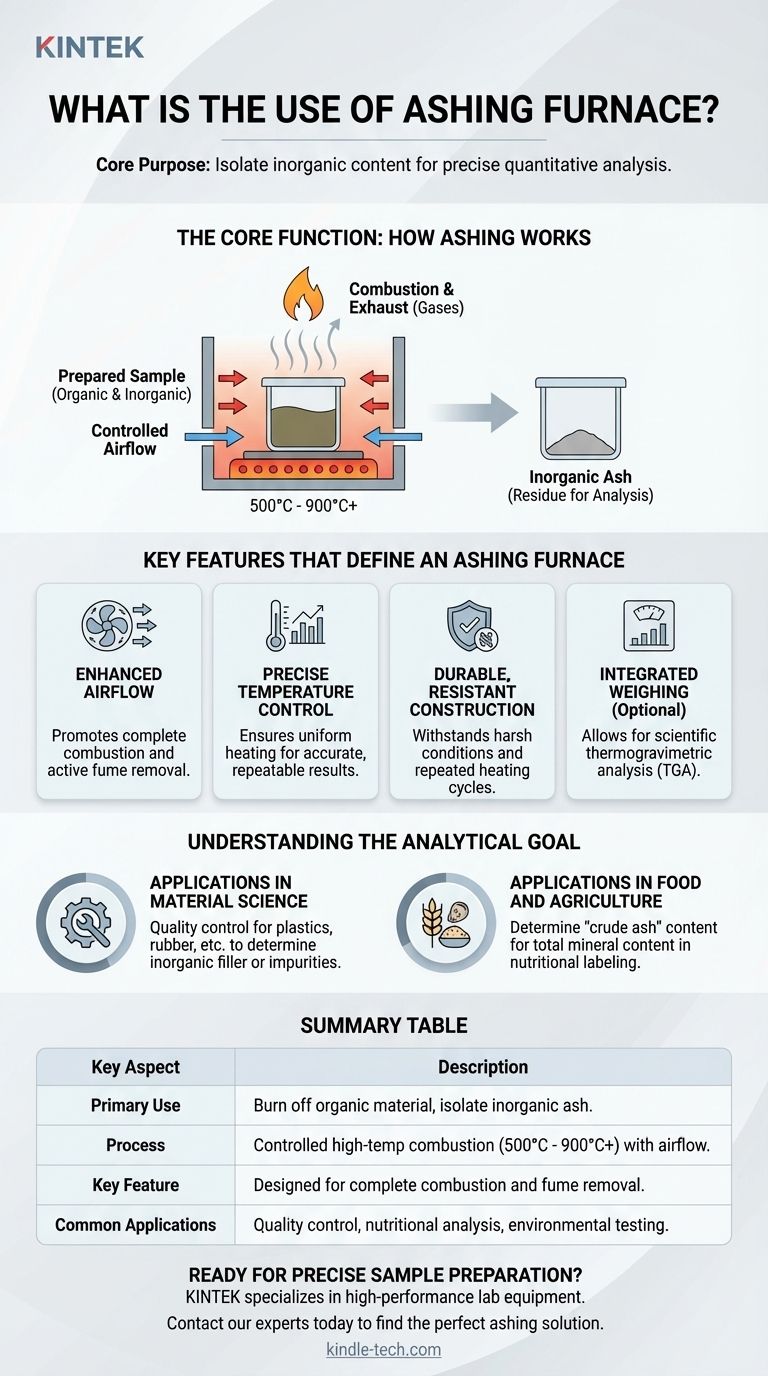
The Core Function: How Ashing Works
An ashing furnace executes a controlled process called incineration or "ashing." It is designed to ensure this process is complete and repeatable, providing a reliable basis for analysis.
The Principle of Combustion
The furnace heats a prepared sample in a chamber with a controlled supply of air. The high temperature, typically ranging from 500°C to over 900°C, causes the organic compounds in the sample to react with oxygen and combust.
Removing Organic Material
During combustion, the organic components—primarily carbon-based molecules—are converted into gases like carbon dioxide and water vapor. These volatile substances are then safely exhausted from the chamber.
Isolating the Inorganic Ash
What remains after complete combustion is the ash. This residue consists of the inorganic compounds originally present in the sample, such as minerals, salts, and metallic oxides. This ash is the true target of the analysis.
Key Features That Define an Ashing Furnace
While it may resemble a standard muffle furnace, an ashing furnace is specifically designed to facilitate complete and efficient combustion.
Enhanced Airflow
This is a critical feature. Ashing furnaces are engineered to promote a high rate of airflow through the heating chamber. This constant supply of fresh oxygen is vital for ensuring the sample combusts completely and for actively removing the smoke and fumes created during the process.
Precise Temperature Control
To yield accurate and repeatable results, the furnace must maintain a uniform temperature throughout the chamber. Different materials require specific temperature profiles to ensure all organic matter is burned off without vaporizing any of the target inorganic compounds.
Durable, Resistant Construction
The combustion process can release aggressive or corrosive byproducts. The interior chamber of an ashing furnace is therefore built from materials that can withstand these harsh conditions and repeated heating cycles over a long service life.
Integrated Weighing (Optional)
Some advanced ashing furnaces include a built-in scale. This allows for the scientific weighing of a sample before, during, and after combustion, a process known as thermogravimetric analysis (TGA), to determine precisely how the mass changes with temperature.
Understanding the Analytical Goal
The purpose of ashing is almost always to answer a quantitative question: "What percentage of this material is inorganic filler?" or "What is the total mineral content of this food product?"
Applications in Material Science
In industries that produce plastics, rubber, or coal, ashing is a routine quality control method. It is used to determine the amount of inorganic filler or impurities, ensuring the final product meets its required specifications for properties like strength, density, or purity.
Applications in Food and Agriculture
When analyzing grain, feed, or other food products, ashing is used to determine the "crude ash" content. This figure represents the total mineral content, a key metric for nutritional labeling and quality assessment.
Making the Right Choice for Your Goal
The ultimate goal of your analysis dictates how you use the results from an ashing furnace.
- If your primary focus is quality control for materials like plastic or rubber: Use ashing to verify that the percentage of inorganic filler matches the product's design specification.
- If your primary focus is nutritional analysis for food or agricultural products: Use ashing to determine the total mineral content, a critical component of a complete nutritional profile.
- If your primary focus is environmental analysis: Use ashing to reduce a sample (like soil or sludge) to its inorganic components, which can then be tested for heavy metals or other contaminants.
Ultimately, the ashing furnace is a foundational tool for revealing the hidden inorganic composition of a material.
Summary Table:
| Key Aspect | Description |
|---|---|
| Primary Use | To burn off organic material and isolate inorganic ash for quantitative analysis. |
| Process | Controlled high-temperature combustion (500°C - 900°C+) with enhanced airflow. |
| Key Feature | Designed for complete combustion and fume removal. |
| Common Applications | Quality control (plastics, rubber), nutritional analysis (food), environmental testing. |
Ready to achieve precise sample preparation and reliable inorganic analysis?
KINTEK specializes in high-performance lab equipment, including ashing furnaces designed for accurate, repeatable results in quality control and material science.
Contact our experts today to find the perfect ashing solution for your laboratory's needs.
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the debinding process? A Guide to Critical Binder Removal for MIM & 3D Printing
- How do you cool a muffle furnace? Safeguard your equipment and samples from thermal shock.
- What is the effect of temperature on calcination? Master Precise Heat Control for Material Properties
- What are the working principles of furnace? A Guide to Combustion, Resistance, and Induction Heating
- What is the difference between a box furnace and a muffle furnace? Choose the Right Lab Furnace for Your Application



















