Yes, you can absolutely braze two different metals. In fact, joining dissimilar materials is one of the primary strengths of the brazing process. This method allows you to join a vast array of metals—including steels, copper, nickel, aluminum, and even ceramics—by using a filler metal that melts at a lower temperature than the two base materials being joined.
The core principle is straightforward: while almost any two metals can be brazed, the success of the joint depends entirely on the filler metal's ability to "wet" and bond with both surfaces, a process that requires meticulous cleaning and control of surface oxides.
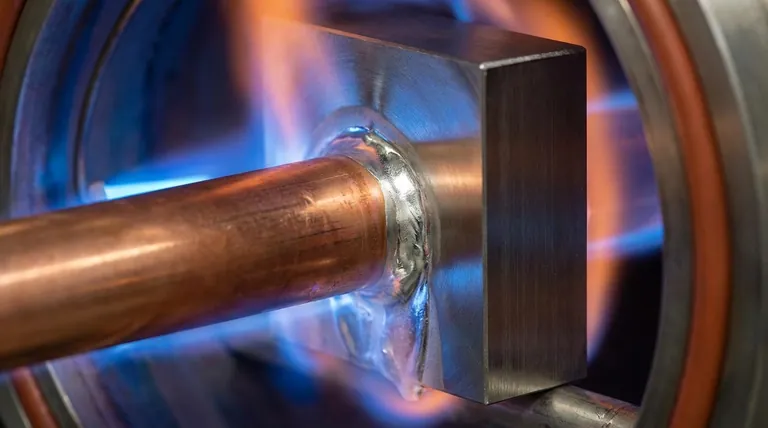
The Fundamental Principle: "Wetting"
Brazing works by capillary action, where the molten filler metal is drawn into the tight gap between the two base materials. For this to happen, the filler must be able to "wet" the surfaces.
What is Wetting?
Think of wetting as the way water spreads out on a clean glass plate versus how it beads up on a waxed car. For a strong braze joint, the molten filler metal must spread smoothly across both base metal surfaces, adhering to them completely.
The Role of Surface Oxides
Almost all metals form an invisible layer of oxide when exposed to air. This oxide layer acts like a barrier, preventing the filler metal from making direct contact with the base metal and thus preventing wetting.
Overcoming the Oxide Barrier
To achieve a successful braze, you must remove and prevent this oxide layer from re-forming during the heating process. This is accomplished in two primary ways:
- Flux: A chemical compound applied to the joint area before heating. The flux melts, dissolves the oxides, and shields the joint from the atmosphere.
- Controlled Atmosphere: Brazing is performed inside a furnace filled with a specific gas (like hydrogen) or under a vacuum. This atmosphere chemically removes oxides or prevents them from forming in the first place.
Common Dissimilar Metal Combinations
Brazing is exceptionally versatile, but some combinations are more straightforward than others.
Steel, Copper, and Nickel Alloys
These materials are commonly brazed to one another with a high degree of success. Their oxides are relatively easy to manage, and many standard filler metals (often silver or copper-based alloys) are compatible with them.
The Challenge of Aluminum
Aluminum can be brazed to other metals like titanium, nickel, and beryllium. However, its tenacious oxide layer and low melting point require special fluxes and precise temperature control.
Notably, aluminum cannot be directly brazed to copper or brass using standard techniques. These combinations require special measures, such as pre-plating one of the metals with a more compatible material like nickel.
Advanced Materials: Ceramics and Refractory Metals
The principles of brazing even extend to non-metals. Ceramics can be brazed to metals, provided the filler alloy can wet both surfaces. Refractory metals (like tungsten) are often joined to reactive metals (like titanium) using specialized vacuum brazing techniques to prevent any contamination.
Understanding the Key Considerations
Joining two different metals introduces complexities that must be managed for a reliable joint.
Filler Metal Compatibility
The filler metal is the bridge between the two materials. Its melting point must be lower than both base metals to ensure they do not melt during the process. It must also be chemically compatible with both to form a strong metallurgical bond.
Managing Thermal Expansion
Different materials expand and contract at different rates when heated and cooled. If this difference (the coefficient of thermal expansion) is significant, it can create immense stress on the joint as it cools, potentially causing it to crack or fail. This must be managed through process design and heating/cooling rates.
Avoiding Galvanic Corrosion
When two dissimilar metals are in contact in the presence of an electrolyte (like moisture), they can form a galvanic cell, causing one of the metals to corrode rapidly. The choice of filler metal can influence this effect, so it's a critical consideration for parts intended for long-term service in certain environments.
Making the Right Choice for Your Goal
Your approach depends entirely on the materials you need to join and the performance you require.
- If your primary focus is general fabrication (e.g., steel to copper): A standard silver-based filler metal and a chemical flux are often sufficient for a strong bond.
- If your primary focus is joining reactive metals (e.g., aluminum to titanium): You will need a specialized flux designed for aluminum or a controlled atmosphere furnace, along with a compatible filler alloy.
- If your primary focus is high-performance applications (e.g., refractory metals): These demanding joints almost always require specialized vacuum furnace brazing to ensure purity and joint integrity.
By understanding these core principles, you can confidently use brazing to create strong, reliable joints between a vast range of different materials.
Summary Table:
| Key Consideration | Why It Matters for Dissimilar Metals |
|---|---|
| Filler Metal Wetting | The filler must flow and bond with both metal surfaces for a strong joint. |
| Thermal Expansion | Different expansion rates can cause stress; heating/cooling must be controlled. |
| Galvanic Corrosion | Dissimilar metals in contact can corrode; filler choice can mitigate this risk. |
| Oxide Removal | Surface oxides must be removed using flux or a controlled atmosphere (e.g., vacuum). |
Need to create a strong, reliable joint between dissimilar metals? The right equipment is critical for controlling the brazing atmosphere and temperature. KINTEK specializes in lab equipment and consumables, including brazing furnaces, to help you achieve perfect results. Let our experts help you select the ideal solution for your specific materials and application. Contact us today for a consultation!
Visual Guide

Related Products
- Vacuum Heat Treat Sintering Brazing Furnace
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
People Also Ask
- What is the most important factor influencing the strength of the brazed joint? Master Joint Clearance for Maximum Strength
- How is the greatest joint strength obtained in brazing? Master the 3 Keys to Superior Metallurgical Bonds
- Where are vacuum furnaces used? Essential for High-Purity Heat Treatment in Critical Industries
- What are the factors that affect the strength of a brazed joint? Master the 4 Keys to a Perfect Bond
- What are the advantages of brazing over braze welding? Achieve Stronger, Cleaner, and Repeatable Joints



















