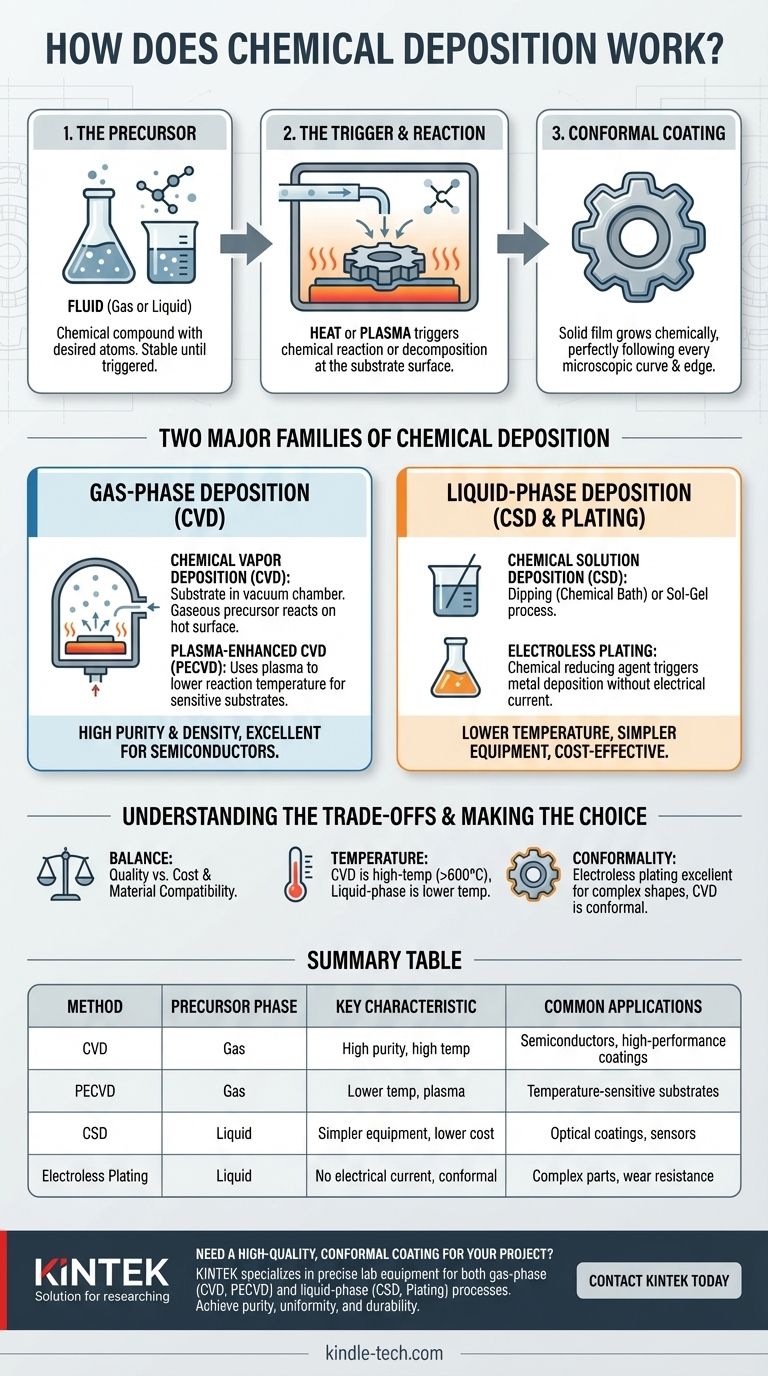
At its core, chemical deposition is a process where a fluid—either a gas or a liquid—undergoes a chemical reaction on the surface of an object to create a solid, thin film. The key is that the new layer is not simply applied, but is chemically formed directly onto the part, resulting in a highly uniform and adherent coating.
Chemical deposition is not a single method but a family of techniques used to grow thin films. The defining principle is using a chemical reaction at a surface to create a solid layer. The primary difference between methods lies in the state of the starting material, known as the precursor: gas or liquid.

The Fundamental Principle: From Fluid to Solid Film
Chemical deposition is a foundational process in materials science and manufacturing, used to create everything from semiconductor chips to wear-resistant coatings on tools. The principle remains consistent across all its variations.
The Role of the Precursor
Every chemical deposition process starts with a precursor. This is a chemical compound in a fluid state (gas or liquid) that contains the atoms you want to deposit.
The precursor is designed to be stable at room temperature but reactive under specific conditions.
The Trigger for Reaction
The precursor fluid is introduced to a chamber containing the object to be coated, known as the substrate. The process is then triggered, most commonly by heat.
When the substrate is heated to a specific reaction temperature, the precursor molecules that come into contact with its hot surface react or decompose. This chemical change "breaks apart" the precursor, leaving the desired solid material bonded to the surface.
The Hallmark of Conformal Coating
A major advantage of chemical deposition is its ability to produce conformal films. This means the coating grows at an even thickness across the entire exposed surface of the object.
It perfectly follows every microscopic curve, edge, and internal feature, unlike line-of-sight processes (like spray painting) that cannot coat hidden areas.
The Two Major Families of Chemical Deposition
The specific technique used depends on the precursor's phase. This splits the field into two main categories: gas-phase and liquid-phase deposition.
Gas-Phase Deposition: Chemical Vapor Deposition (CVD)
Chemical Vapor Deposition (CVD) is the most prominent gas-phase technique. The substrate is placed in a reaction chamber under a controlled vacuum.
A volatile, gaseous precursor is then introduced into the chamber. The vacuum ensures a pure environment and controls the pressure, allowing the gas to fill the entire space.
When the substrate is heated, the gas precursor reacts on its surface, building the solid film one atomic layer at a time. This results in exceptionally pure and uniform coatings. A common variation, Plasma-Enhanced CVD (PECVD), uses plasma to energize the gas, allowing the reaction to occur at much lower temperatures.
Liquid-Phase Deposition: CSD & Plating
This category uses a precursor dissolved in a liquid solvent. The methods are generally simpler and operate at lower temperatures than traditional CVD.
Chemical Solution Deposition (CSD) involves techniques like dipping the substrate into a chemical bath (Chemical Bath Deposition) or using a Sol-Gel process where the liquid solidifies into a gel and is then heat-treated.
Plating is another common liquid-phase method. Electroless Plating, for example, uses a chemical reducing agent within the bath to trigger the deposition of metal onto the substrate without any external electrical current.
Understanding the Trade-offs
No single method is universally superior. The choice involves balancing the need for quality, material compatibility, and cost.
CVD: Strengths and Limitations
The strength of CVD is its ability to produce extremely high-purity, dense, and conformal films, making it the standard for high-performance applications like semiconductors.
However, the high temperatures required (often >600°C) can damage sensitive substrates like plastics or certain metals. The process also requires complex and expensive vacuum equipment.
CSD & Plating: Strengths and Limitations
Liquid-phase methods are attractive because they operate at or near room temperature and typically require simpler, less costly equipment.
The primary trade-off is often film purity and density. The coatings can sometimes incorporate impurities from the solvent, and they may not achieve the same level of atomic perfection as films grown via CVD.
Making the Right Choice for Your Goal
Selecting the correct method requires aligning the process capabilities with your material and performance requirements.
- If your primary focus is ultimate purity and uniformity for high-performance electronics: High-temperature CVD is the industry standard for creating flawless thin films.
- If you are working with temperature-sensitive materials like polymers or pre-built devices: PECVD or a liquid-phase method like CSD offers a low-temperature alternative.
- If your goal is a cost-effective and durable metal coating on complex shapes: Electroless plating provides excellent conformal coverage without complex vacuum systems.
Ultimately, understanding the interplay between the precursor, the reaction trigger, and the substrate empowers you to select the ideal deposition technique for your specific application.
Summary Table:
| Method | Precursor Phase | Key Characteristic | Common Applications |
|---|---|---|---|
| CVD (Chemical Vapor Deposition) | Gas | High purity, high temperature (>600°C) | Semiconductors, high-performance coatings |
| PECVD (Plasma-Enhanced CVD) | Gas | Lower temperature, uses plasma | Temperature-sensitive substrates |
| CSD (Chemical Solution Deposition) | Liquid | Simpler equipment, lower cost | Optical coatings, sensors |
| Electroless Plating | Liquid | No electrical current, conformal coating | Complex parts, wear resistance |
Need a High-Quality, Conformal Coating for Your Project?
Choosing the right chemical deposition method is critical for the performance of your materials. KINTEK specializes in providing the precise lab equipment and consumables needed for both gas-phase (CVD, PECVD) and liquid-phase (CSD, Plating) deposition processes. Our expertise ensures you achieve the purity, uniformity, and durability your application demands.
Let our experts help you select the ideal solution. Contact KINTEK today to discuss your specific laboratory needs!
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Laboratory CVD Boron Doped Diamond Materials
People Also Ask
- What are different types of thin films? A Guide to Function, Material, and Deposition Methods
- What is the difference between PECVD and APCVD? Choose the Right CVD Method for Your Application
- Why does a PECVD vacuum system require both a rotary vane and turbo pump? Ensure High-Purity Coatings
- How do PECVD systems improve DLC coatings on implants? Superior Durability and Biocompatibility Explained
- What is the process of PECVD in semiconductor? Enabling Low-Temperature Thin Film Deposition



















