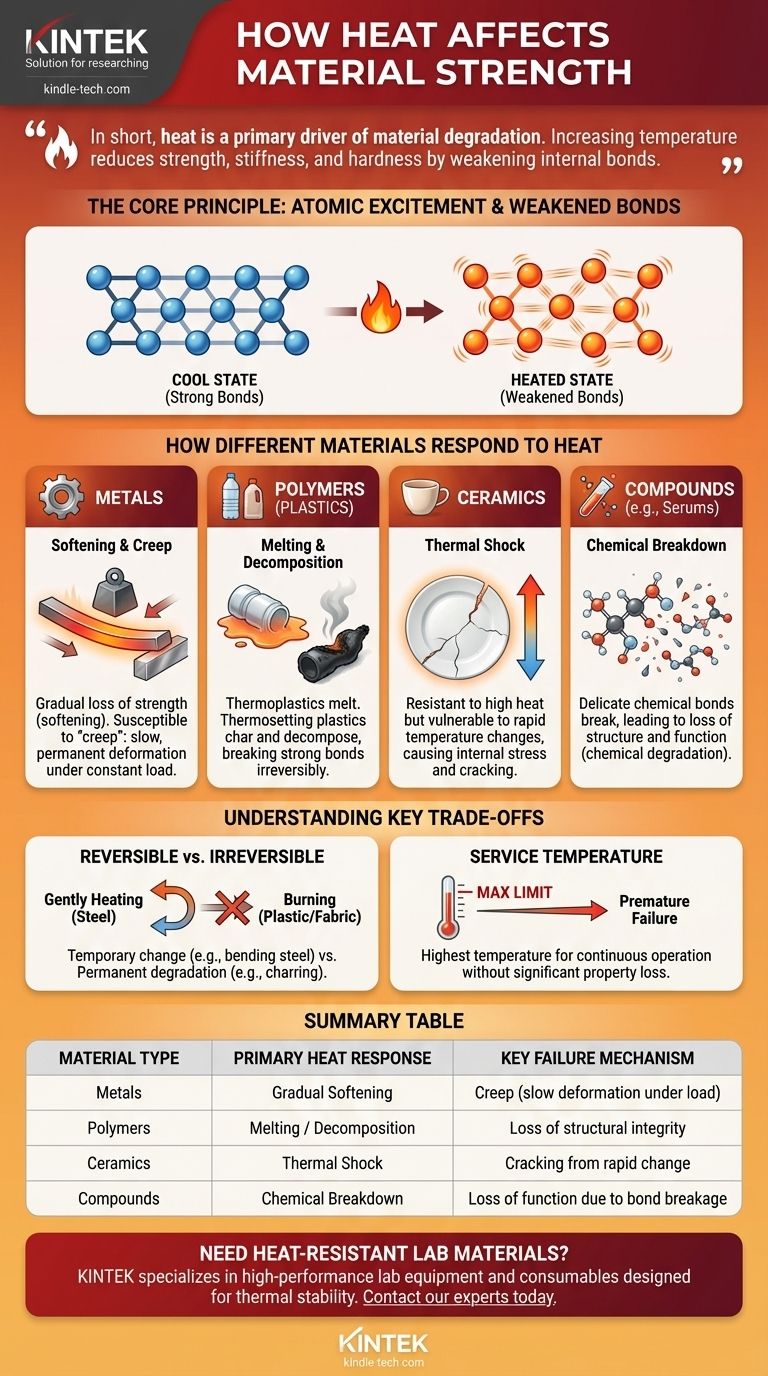
In short, heat is a primary driver of material degradation. For most materials, increasing temperature reduces strength, stiffness, and hardness by weakening the internal bonds that hold the structure together. This can manifest as gradual softening in metals, melting in plastics, or even the complete chemical decomposition of organic compounds and fabrics.
The core issue is that heat introduces energy into a material's atomic structure. This increased energy causes atoms to vibrate more intensely, pushing them apart and weakening the forces that provide structural integrity, ultimately leading to failure.

The Fundamental Principle: Atomic Excitement and Weakened Bonds
How Temperature Affects Atomic Structure
Heat is simply a form of energy. When you apply heat to a material, its atoms and molecules absorb that energy and begin to vibrate more rapidly and intensely.
The Impact on Material Bonds
This constant, vigorous vibration increases the average distance between atoms. As the atoms move farther apart, the interatomic and intermolecular forces that hold them together in a rigid structure become weaker, directly reducing the material's overall strength and stiffness.
How Different Material Classes Respond to Heat
The specific way a material fails under heat depends entirely on its internal structure. Metals, polymers, and ceramics each behave differently.
Metals: Softening and Creep
Metals typically do not fail suddenly when heated. Instead, they lose strength gradually in a process called softening, where properties like yield strength and hardness decline.
At high temperatures, metals also become susceptible to creep, a slow and permanent deformation that occurs under a constant load, even if that load is well below its normal yield strength. This is why a sharp steel edge can become dull when heated repeatedly; the metal loses its hardness.
Polymers (Plastics): Melting and Decomposition
Polymers have a much lower tolerance for heat than metals. Thermoplastics, like the plasticware in an autoclave, have long molecular chains that are not chemically bonded to each other. Heat allows these chains to slide past one another, causing the material to soften and eventually melt into a liquid.
Thermosetting plastics, on the other hand, have a cross-linked chemical structure. They do not melt but will begin to char and decompose at high temperatures as the heat becomes intense enough to break these strong chemical bonds. The destruction of fabrics and linens is another example of this decomposition.
Ceramics: Thermal Shock
While ceramics are extremely resistant to high temperatures, their primary weakness is thermal shock. Their rigid, crystalline structure does not expand or contract uniformly or quickly.
A rapid change in temperature can create internal stresses that exceed the material's strength, causing it to crack and fail catastrophically.
Compounds and Solutions: Chemical Breakdown
For complex molecules like those found in proteins, serums, or vaccines, heat can be enough to break the delicate chemical bonds that give the compound its structure and function. This is not a loss of mechanical strength but a chemical degradation that renders the compound useless.
Understanding the Key Trade-offs
Reversible vs. Irreversible Changes
It's critical to distinguish between temporary and permanent damage. Gently heating a piece of steel to bend it is a reversible change in properties; it will regain most of its strength upon cooling.
In contrast, burning a piece of plastic or charring fabric is an irreversible chemical change. The material is permanently degraded and cannot be returned to its original state.
The Concept of Service Temperature
Every engineering material has a maximum service temperature. This is the highest temperature at which it can operate continuously without a significant or unacceptable loss of its mechanical properties. Exceeding this limit leads to premature failure.
The Role of Oxidation
Heat dramatically accelerates chemical reactions, including oxidation. For many metals, like iron and steel, high temperatures in the presence of oxygen will rapidly form a weak, brittle layer of oxide (rust), compromising the integrity of the material from the outside in.
Making the Right Choice for Your Goal
When selecting a material, you must match its thermal properties to the demands of the operating environment.
- If your primary focus is extreme-temperature strength: Refractory metals and technical ceramics are the clear choice, but you must design to mitigate ceramic brittleness and potential for thermal shock.
- If your primary focus is a balance of strength and cost for moderate temperatures: Standard metals like steel and aluminum or high-performance polymers offer excellent performance, but their strength will predictably decrease as temperatures rise.
- If your primary focus is on lightweight applications without significant heat: Commodity plastics are effective, but they possess very low melting points and should never be used where temperatures are a concern.
Ultimately, managing the effects of heat is about understanding a material's specific thermal limits and designing within those constraints to ensure safety and reliability.
Summary Table:
| Material Type | Primary Heat Response | Key Failure Mechanism |
|---|---|---|
| Metals | Gradual Softening | Creep (slow deformation under load) |
| Polymers (Plastics) | Melting or Decomposition | Loss of structural integrity |
| Ceramics | Thermal Shock | Cracking from rapid temperature change |
| Compounds (e.g., Serums) | Chemical Breakdown | Loss of function due to bond breakage |
Need materials that can withstand high temperatures in your lab? KINTEK specializes in high-performance lab equipment and consumables designed for thermal stability. Whether you're working with heat-sensitive samples or high-temperature processes, our solutions ensure reliability and precision. Contact our experts today to find the right equipment for your laboratory's thermal challenges!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- Which gas is used as a carrier gas for Al deposition using the sputtering system? Argon is the Standard for Pure Aluminum Films
- What is the difference between cold type and hot type? Uncover the Printing Revolution
- Which element made stainless steel difficult to brazed? It's Chromium's Oxide Layer
- What are the most common elemental analysis techniques? Choose the Right Tool for Your Material Analysis
- What is the temperature of pyrolysis products? Control the Heat to Control Your Output
- Is it fitting the mould or mold? A Guide to Correct Spelling by Region
- What are the basic components of heat treatment? Mastering the 3 Stages for Superior Material Properties
- What is the function of a crucible furnace? A Guide to Controlled Melting for Casting and Alloying



















