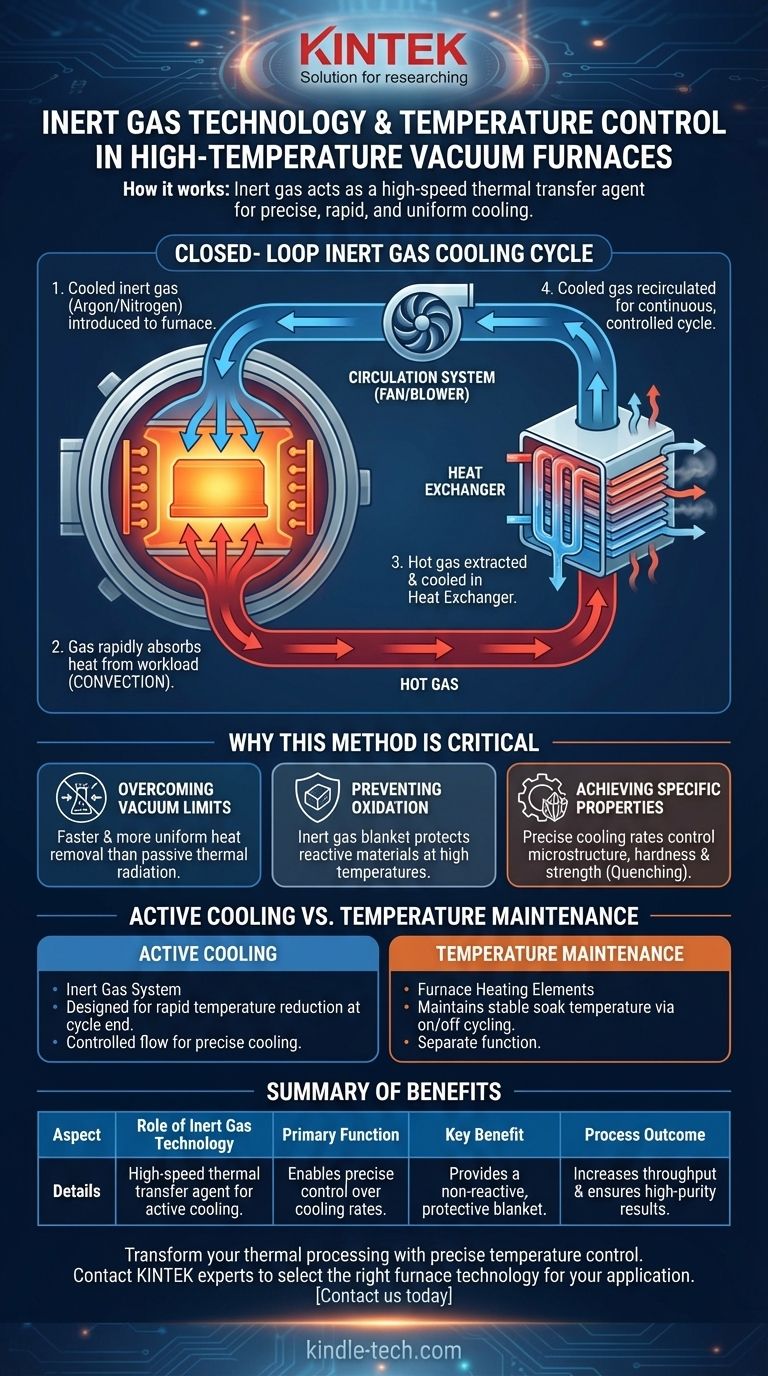
In short, inert gas technology controls temperature by acting as a high-speed thermal transfer agent. A circulation system introduces cooled inert gas into the furnace's hot zone. This gas rapidly absorbs heat from the workload through convection and is then extracted, cooled by a heat exchanger, and recirculated, creating a continuous and precisely controlled cooling cycle.
The core function of inert gas in a high-temperature furnace is not just to prevent oxidation, but to enable rapid, uniform, and controllable cooling—a feat impossible to achieve through radiation in a vacuum or with reactive air.

The Mechanics of Forced Convection Cooling
To understand how this works, it's best to think of the inert gas as a conveyor belt for heat. The system is designed to move heat out of the furnace chamber as efficiently as possible.
The Key Components
The system relies on three critical elements working in concert:
- Inert Gas: Typically Argon or Nitrogen, chosen because it will not chemically react with the hot workload. It serves as the medium for heat transfer.
- Heat Exchanger: This device, often water-cooled, is the destination for the heat. It strips the thermal energy from the gas before it's sent back into the furnace.
- Circulation System: A powerful fan or blower is used to move the inert gas through this closed loop, from the heat exchanger into the furnace and back again.
The Cooling Cycle Step-by-Step
The process is a continuous loop. Once the heating cycle is complete and cooling needs to begin, the system is activated.
The cooled inert gas is forced into the hot chamber. It flows over the product and the furnace's refractory materials, absorbing their thermal energy far more effectively than passive radiation.
This now-hot gas is immediately drawn out of the chamber and directed through the heat exchanger, where its heat is transferred away (e.g., into cooling water).
The now-cooled gas is then pushed back into the furnace to absorb more heat. The speed of this cycle is managed by a valve that controls the gas flow rate, giving operators precise control over the cooling curve.
Why This Method is Critical
Using inert gas isn't just an option; for many advanced material processes, it's a requirement. The reasons go far beyond simple temperature reduction.
Overcoming the Limits of a Vacuum
In a pure vacuum, the only way for an object to cool is through thermal radiation. This process is slow, non-uniform, and becomes less efficient as the object cools. Inert gas introduces forced convection, a dramatically faster and more even method of heat removal.
Preventing Oxidation and Contamination
At high temperatures, many advanced materials, metals, and alloys are highly reactive with oxygen. Introducing air would instantly cause destructive oxidation. An inert gas provides a protective, non-reactive blanket around the workload throughout the entire heating and cooling process.
Achieving Specific Material Properties
The final microstructure and physical properties (like hardness and strength) of many alloys are determined by the rate at which they are cooled. This controlled cooling, often called quenching, is only possible when you can precisely manage the speed of heat extraction. The variable flow rate of an inert gas system provides this exact capability.
Understanding the System's Role
It is crucial to distinguish between two different temperature control functions in a furnace.
Active Cooling vs. Temperature Maintenance
The inert gas system is an active cooling mechanism designed for rapid temperature reduction at the end of a cycle.
The furnace's primary temperature control for holding a setpoint is different. As described by simple on/off indicators, that system energizes and de-energizes the heating elements to maintain a stable temperature during the soak phase. These are two separate systems for two different jobs.
Inherent Trade-offs
The primary trade-off is complexity and cost. An inert gas quenching system requires a powerful, sealed circulation fan, extensive plumbing, a large heat exchanger, and a supply of high-purity gas. This adds significant capital and operational expense compared to a furnace that only cools passively.
Making the Right Choice for Your Goal
Selecting a furnace with inert gas cooling depends entirely on the requirements of your process.
- If your primary focus is high-purity, oxygen-free processing: An inert gas atmosphere is essential to prevent oxidation and contamination during the entire thermal cycle.
- If your primary focus is production throughput: Inert gas quenching can reduce cooling times from many hours to under an hour, dramatically increasing the number of cycles a furnace can run per day.
- If your primary focus is achieving specific metallurgical properties: The precise, adjustable cooling rates provided by a variable-flow gas system are non-negotiable for processes like hardening, annealing, or brazing that depend on a specific cooling curve.
Ultimately, inert gas technology gives you active command over the thermal environment, transforming the cooling phase from a passive wait into a controlled and critical process step.
Summary Table:
| Aspect | Role of Inert Gas Technology |
|---|---|
| Primary Function | Acts as a high-speed thermal transfer agent for active cooling. |
| Key Benefit | Enables precise control over cooling rates for specific material properties. |
| Atmosphere | Provides a non-reactive, protective blanket to prevent oxidation. |
| Process Outcome | Increases production throughput and ensures high-purity results. |
Ready to transform your thermal processing with precise temperature control?
At KINTEK, we specialize in advanced laboratory equipment, including high-temperature vacuum furnaces with inert gas quenching systems. Whether your goal is to achieve specific metallurgical properties, increase production throughput, or ensure contamination-free processing, our solutions are designed to meet your exact needs.
Let our experts help you select the right furnace technology for your application. Contact us today to discuss your project and discover how KINTEK can enhance your lab's capabilities and efficiency.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Brazing Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What materials are used in a vacuum furnace? Selecting the Right Hot Zone for Your Process
- What are the advantages of vacuum hardening? Achieve Superior Precision and Cleanliness for Critical Components
- What is the standard thickness of plating? Optimize Durability, Corrosion & Cost
- What is the process of a vacuum furnace? Achieve Purity and Precision in High-Temp Processing
- What are vacuum furnaces used for? Unlock Ultimate Material Purity and Performance



















