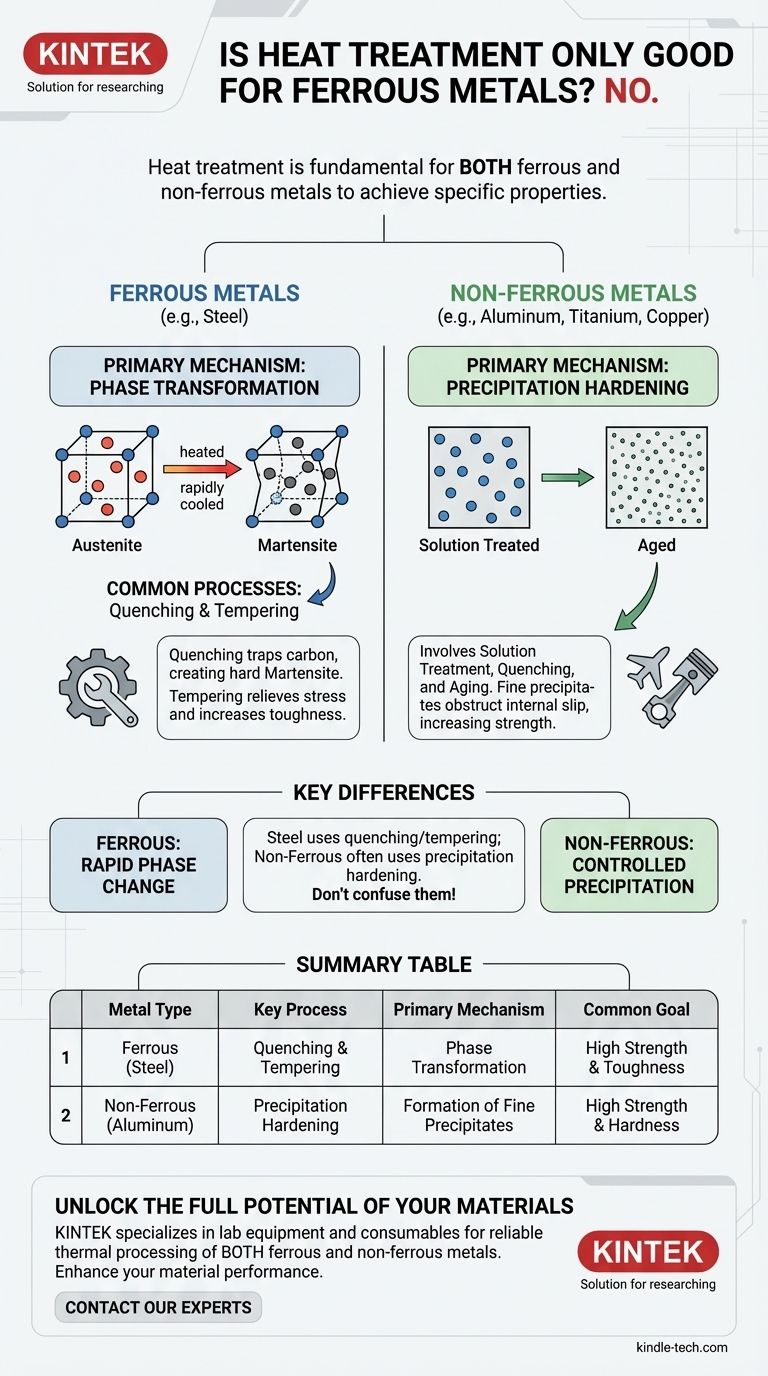
No, absolutely not. Heat treatment is a fundamental metallurgical practice applied to both ferrous and non-ferrous metals to achieve specific properties. While processes like quenching and tempering are famously associated with steel, a vast range of non-ferrous alloys, including aluminum, titanium, and copper, rely on distinct heat treatment methods to unlock their full performance potential.
The core principle of heat treatment—using controlled heating and cooling to manipulate a metal's internal microstructure—is universal. However, the specific metallurgical mechanisms are fundamentally different: ferrous metals primarily rely on phase transformations, while many non-ferrous alloys depend on precipitation hardening.

The Universal Goal: Controlling Microstructure
Heat treatment is not a single process but a family of techniques. The shared goal is to intentionally alter the physical, and sometimes chemical, properties of a material to make it more suitable for its intended application.
Why Microstructure is Everything
A metal’s performance characteristics—its strength, hardness, ductility, and toughness—are a direct result of its internal crystal structure, known as its microstructure.
By applying heat and controlling the rate of cooling, we can change the size, shape, and distribution of the crystals and phases within the metal, thereby tailoring its final properties.
How Heat Treatment Works on Ferrous Metals (Steel)
For ferrous metals like steel, heat treatment is almost entirely about controlling the relationship between iron and carbon.
The Role of Carbon and Phase Transformation
Heating steel to a high temperature transforms its crystal structure into a phase called austenite, which can dissolve a significant amount of carbon.
When this austenite is cooled rapidly (quenched), the carbon atoms are trapped, creating a very hard, brittle, and distorted structure called martensite.
Common Processes: Quenching and Tempering
Quenching is the process that creates the hard martensitic structure. However, this structure is often too brittle for practical use.
Tempering is a subsequent, lower-temperature heating process that relieves internal stresses and slightly reorganizes the microstructure to increase toughness and ductility, albeit at a slight cost to maximum hardness.
Surface Treatments: Case Hardening
Processes like carburising are specific to steel. They involve diffusing carbon into the surface of a low-carbon steel part at high temperature, creating a component with a very hard, wear-resistant surface (the "case") and a tough, ductile core.
How Heat Treatment Works on Non-Ferrous Metals
Many non-ferrous alloys cannot form martensite and thus do not respond to quenching and tempering in the same way as steel. Instead, they often rely on a different mechanism.
The Principle of Precipitation Hardening
The most common heat treatment for high-strength aluminum, titanium, and copper alloys is precipitation hardening, also known as age hardening.
This is a multi-step process:
- Solution Treatment: The metal is heated to a high temperature to dissolve alloying elements into a uniform solid solution.
- Quenching: It is then rapidly cooled, trapping these elements in a supersaturated state.
- Aging: The metal is reheated to a lower temperature for an extended period. This allows the alloying elements to precipitate out of the solution as extremely fine, dispersed particles that obstruct internal slip and dramatically increase strength and hardness.
Example: Aluminum Alloys
A common aluminum alloy like 6061-T6 gets its strength from this exact process. The "-T6" temper designation specifically signifies that it has been solution heat-treated and then artificially aged.
Understanding the Key Differences
Confusing the heat treatment principles for ferrous and non-ferrous metals is a common and critical error. The underlying metallurgy is fundamentally distinct.
Phase Transformation vs. Precipitation
The key takeaway is the difference in mechanism. Steel hardening is driven by a rapid, diffusionless phase transformation (austenite to martensite). In contrast, aluminum hardening is driven by the controlled, time-and-temperature-dependent precipitation of secondary phases.
Why You Can't "Temper" Aluminum Like Steel
The term "temper" for aluminum alloys (e.g., -T4, -T6) refers to its condition of heat treatment, specifically related to the aging process. It is not the same as the tempering process used to toughen hardened steel. Applying a steel tempering cycle to a precipitation-hardened aluminum alloy would likely over-age it, causing the fine precipitates to coarsen and dramatically reducing its strength.
Making the Right Choice for Your Material
Understanding which mechanism is at play is the first step toward successful heat treatment and material selection.
- If your primary focus is carbon or alloy steels: Your heat treatment will revolve around controlling phase transformations through quenching, tempering, annealing, or normalizing.
- If your primary focus is high-strength aluminum, titanium, or copper alloys: Your process will be precipitation hardening, which involves a precise sequence of solution treating, quenching, and aging.
- If you simply need to soften a work-hardened metal: A process called annealing is used for both ferrous and non-ferrous metals, though the specific temperatures and goals differ for each alloy system.
Ultimately, selecting the correct heat treatment is as critical as selecting the right alloy for the job.
Summary Table:
| Metal Type | Key Heat Treatment Process | Primary Mechanism | Common Goal |
|---|---|---|---|
| Ferrous (e.g., Steel) | Quenching & Tempering | Phase Transformation (Austenite to Martensite) | High Strength & Toughness |
| Non-Ferrous (e.g., Aluminum) | Precipitation Hardening | Formation of Fine Precipitates | High Strength & Hardness |
Unlock the Full Potential of Your Materials
Selecting and executing the correct heat treatment is as critical as choosing the right alloy. Whether you're working with high-strength steel or advanced aluminum alloys, the right equipment and expertise are essential for achieving the desired material properties.
KINTEK specializes in lab equipment and consumables, serving the precise needs of laboratories and R&D facilities. We provide the reliable thermal processing solutions you need to ensure consistent, repeatable results for both ferrous and non-ferrous metals.
Let us help you enhance your material performance. Contact our experts today to discuss your specific application and find the perfect solution for your lab.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
People Also Ask
- What is sputter coating used for? Achieve Superior Thin Films for Electronics, Optics, and Tools
- Why is a magnetic stirrer necessary during the preparation of graphene oxide? Ensure Safety and Uniform Oxidation
- What is the alternative process to sintering? Compare Casting, Forging & Machining for Your Project
- How does microwave sintering work? Achieve Faster, More Uniform Heating for Your Materials
- What apparatus is used for drying specimens? Select the Right Tool to Preserve Your Sample Integrity
- What are the different types of melting process? From Smelting to Suspension for Ultimate Purity
- What are the advantages of using a centrifuge? Achieve Rapid, High-Resolution Sample Separation
- What are the factors affecting the yield of bio-oil from the pyrolysis of coconut shell? Control 4 Key Parameters



















