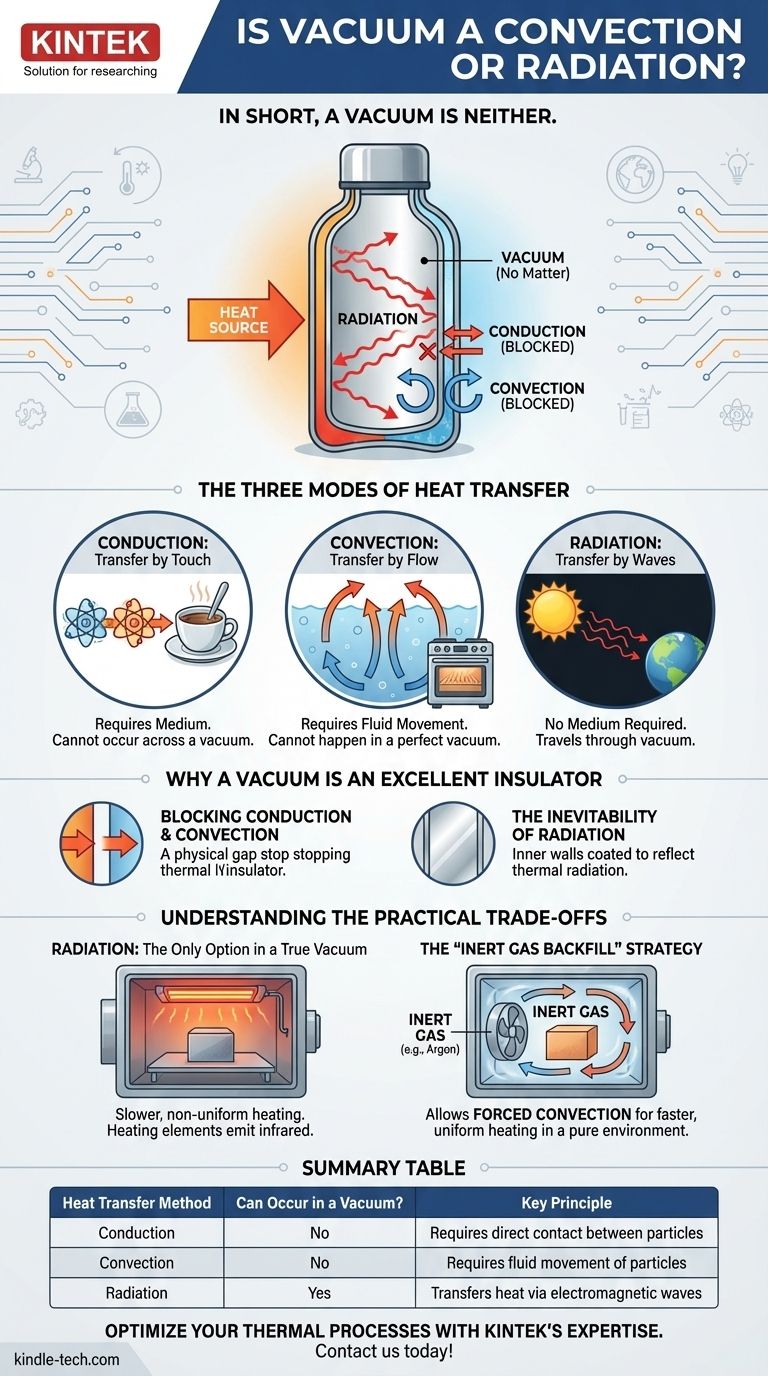
In short, a vacuum is neither. A vacuum is the absence of matter, while convection and radiation are two distinct methods of heat transfer. Because a vacuum lacks the particles needed for conduction or convection, thermal radiation is the only form of heat transfer that can travel through it.
A vacuum acts as a barrier to heat transfer by stopping conduction and convection. This forces any thermal energy exchange to occur solely through electromagnetic waves, a process known as radiation.

The Three Modes of Heat Transfer
To understand why a vacuum behaves this way, we must first distinguish between the three ways heat moves from one place to another.
Conduction: Transfer by Touch
Conduction is the transfer of heat through direct physical contact. Atoms in a hotter material vibrate more rapidly, and when they touch adjacent atoms, they transfer that energy.
This is why a metal spoon in hot coffee becomes hot to the touch. It requires a medium and cannot occur across a vacuum.
Convection: Transfer by Flow
Convection is heat transfer through the bulk movement of fluids (liquids or gases). A warmer, less dense portion of the fluid rises, and the cooler, denser portion sinks, creating a circulating current that distributes heat.
This is how an oven heats food or how a radiator heats a room. As it relies on moving particles, convection cannot happen in a perfect vacuum.
Radiation: Transfer by Waves
Thermal radiation is the transfer of heat via electromagnetic waves, primarily in the infrared spectrum. Unlike conduction and convection, it does not require any medium to travel through.
This is the most fundamental form of heat transfer in the universe. It is how the sun’s warmth travels across the vacuum of space to reach Earth.
Why a Vacuum Is an Excellent Insulator
The unique properties of a vacuum make it an exceptional thermal insulator, a principle used in technologies from coffee flasks to spacecraft.
Blocking Conduction and Convection
By removing the air particles from a space, a vacuum creates a physical gap. This gap effectively stops heat transfer from both conduction (no particles to touch) and convection (no fluid to circulate).
A common thermos, or vacuum flask, uses a double-walled container with a vacuum in between to keep liquids hot or cold for hours.
The Inevitability of Radiation
Even within a vacuum, a hot object will still emit thermal energy as radiation. This is why the inner walls of a high-quality thermos are often coated with a reflective, mirror-like layer.
This silvered surface reflects the thermal radiation back toward the hot liquid (or away from the cold liquid), further slowing heat loss or gain.
Understanding the Practical Trade-offs
While a vacuum is a superb insulator, this has important implications in industrial settings where the goal is often to heat an object quickly and efficiently.
Radiation: The Only Option in a True Vacuum
If you need to heat something inside a pure vacuum, radiation is your only tool. This can be done with heating elements that glow red-hot, emitting intense infrared energy onto the target.
However, relying solely on radiation can be slower than other methods, especially for objects with complex shapes or non-receptive surfaces.
The "Inert Gas Backfill" Strategy
In many industrial processes, such as in a vacuum furnace, the goal is not insulation but controlled heating in a pure environment. First, a vacuum is pulled to remove oxygen and other reactive contaminants.
Then, the chamber is backfilled with a high-purity inert gas, like argon or nitrogen. This clean gas provides a medium for forced convection, allowing fans to circulate the hot gas and heat the product much faster and more uniformly than radiation alone. This is what the term "convection heating after vacuum extraction" refers to.
Making the Right Choice for Your Goal
Your approach depends entirely on whether you want to prevent heat transfer or promote it in a controlled way.
- If your primary focus is insulation: Use a sealed vacuum to create a powerful thermal barrier that stops both conduction and convection.
- If your primary focus is heating in a pure environment: You must rely on thermal radiation from heating elements placed within the vacuum chamber.
- If your primary focus is rapid, uniform heating: Use a vacuum to remove contaminants, then backfill with an inert gas to enable fast and efficient convection.
Understanding this distinction between a medium and a method is the key to mastering any process involving heat and vacuums.
Summary Table:
| Heat Transfer Method | Can Occur in a Vacuum? | Key Principle |
|---|---|---|
| Conduction | No | Requires direct contact between particles |
| Convection | No | Requires fluid movement of particles |
| Radiation | Yes | Transfers heat via electromagnetic waves |
Optimize your thermal processes with KINTEK's expertise in vacuum and heat transfer solutions. Whether you need precise insulation, rapid heating in a pure environment, or uniform temperature control, KINTEK specializes in lab equipment and consumables tailored to your laboratory's unique requirements. Contact us today to discuss how our solutions can enhance your efficiency and results!
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
People Also Ask
- Can a furnace pressure switch cause short cycling? Diagnose the Real Cause of Intermittent Shutdowns
- What role do high-temperature annealing furnaces and water quenching systems play in the post-processing of PM-HIP joints?
- What is the temperature for a furnace? It Depends on Your Material and Process Goal
- Why is a laboratory vacuum drying oven necessary for single-crystal cathode powders? Ensure Peak Material Stability
- What function does the vacuum environment serve during the densification of Ag-SnO2-Y2O3? Optimize Material Density
- Why is 1177 °C precision critical for GH3535 furnace treatment? Ensure Microstructural Integrity
- Why is a laboratory vacuum drying oven recommended for rice straw residues? Protect Your Biomass Integrity
- What is the pressure less sintering method? Achieve Complex Shapes Without High-Pressure Equipment



















