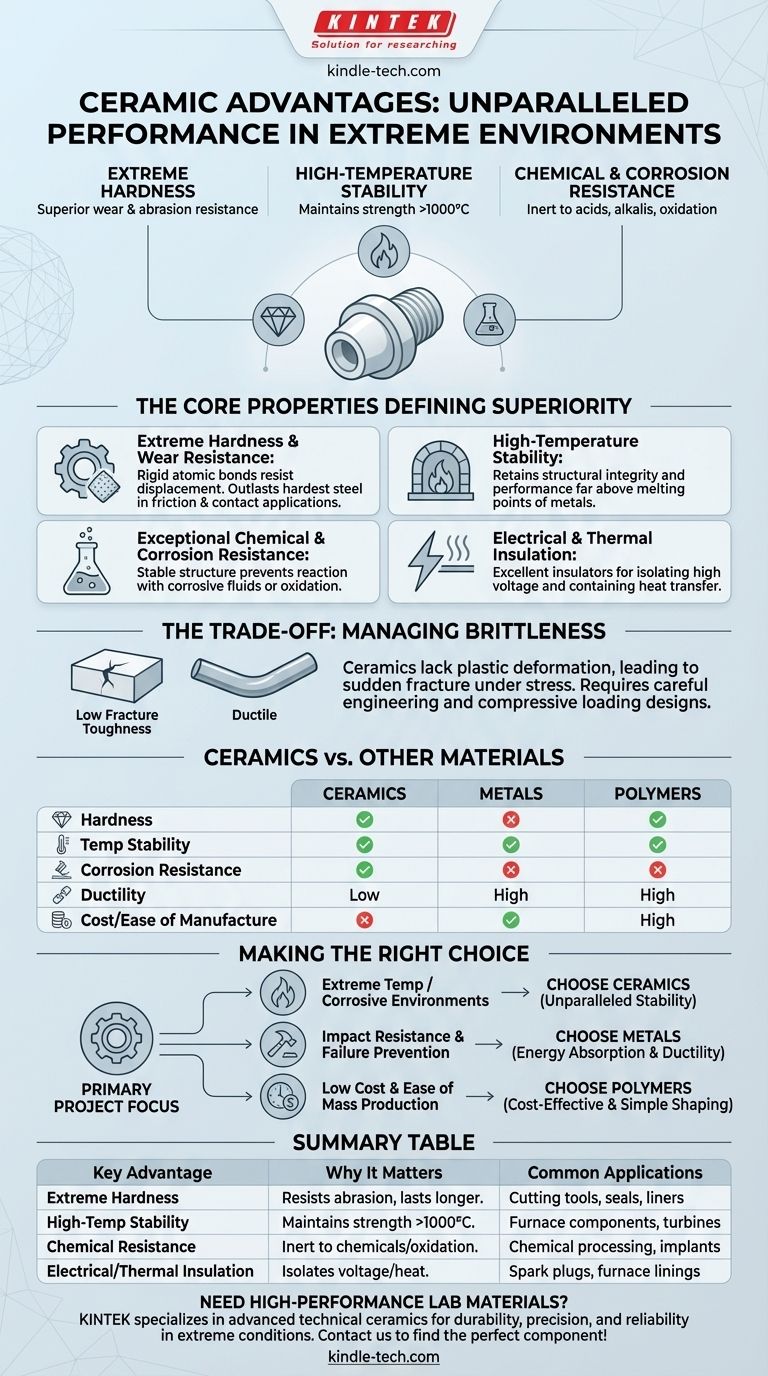
The primary advantages of ceramics are their exceptional hardness, high-temperature stability, and resistance to chemical corrosion. These properties stem from their strong ionic and covalent atomic bonds, making them superior to metals and polymers in extreme environments. Where other materials would wear down, melt, or corrode, advanced technical ceramics maintain their structural integrity and performance.
While often perceived as brittle, advanced ceramics offer a unique combination of extreme hardness, thermal stability, and chemical inertness that is unmatched. Choosing them is about leveraging these specific strengths for demanding applications where ultimate performance is more critical than ductility or cost.

The Core Properties Defining Ceramic Superiority
To understand why ceramics are chosen for some of the most challenging engineering problems, we must look at their fundamental material properties. These are not isolated benefits but interconnected characteristics derived from their atomic structure.
Extreme Hardness and Wear Resistance
Ceramics are among the hardest materials known. The strong, rigid bonds between their atoms resist displacement, making them incredibly difficult to scratch or abrade.
This intrinsic hardness translates directly to outstanding wear resistance. In applications involving friction or contact with abrasive particles, ceramics like silicon carbide and alumina far outlast even the hardest steel alloys.
High-Temperature Stability
Most metals begin to lose their strength, creep, or even melt at temperatures well below 1000°C. In contrast, many ceramics have exceptionally high melting points and can maintain their strength and shape at extreme temperatures.
This thermal stability makes them indispensable as refractory materials in furnaces, as heat shields on spacecraft, and for components inside high-performance engines and gas turbines.
Exceptional Chemical and Corrosion Resistance
The stable atomic structure of ceramics makes them largely inert. They do not react with most acids, alkalis, or organic solvents, and unlike metals, they do not oxidize (rust).
This property is critical for equipment used in chemical processing plants, for seals and pumps handling corrosive fluids, and for biomedical implants that must not react with the human body.
Electrical and Thermal Insulation
While some ceramics are engineered to be conductive, most are excellent electrical insulators. This is why materials like porcelain and alumina are used to isolate high-voltage conductors in power lines and spark plugs.
Furthermore, their atomic structure is not efficient at transferring heat, making them good thermal insulators. This is utilized in everything from furnace linings to the thermal barrier coatings on jet engine turbine blades.
Understanding the Trade-offs: The Challenge of Brittleness
No material is perfect. The same atomic structure that gives ceramics their strengths also creates their primary weakness: brittleness.
Low Fracture Toughness
Metals can bend and deform under stress because their atomic structure allows for dislocations to move. This plastic deformation absorbs energy and prevents catastrophic failure.
Ceramics, with their rigid bonds, have very little capacity for plastic deformation. When subjected to stress beyond their elastic limit—especially tensile stress—they tend to fracture suddenly. This property is known as low fracture toughness.
Impact on Design and Manufacturing
This brittleness must be managed through careful engineering. Designs must avoid sharp corners and stress concentrations. Often, ceramic components are kept under compressive load, which they handle very well.
Machining ceramics is also difficult and expensive. Because of their hardness, they cannot be cut with traditional tools and must be ground with super-hard abrasives like diamond, adding significant cost and complexity to manufacturing.
How Ceramics Compare to Other Material Classes
Choosing a material is always about balancing competing properties. Here is how ceramics stack up directly against metals and polymers.
Ceramics vs. Metals
Ceramics are superior in hardness, high-temperature performance, and corrosion resistance. Metals are far superior in ductility (the ability to deform without breaking) and fracture toughness. Metals are also generally easier and cheaper to machine and form.
Ceramics vs. Polymers (Plastics)
Ceramics vastly outperform polymers in terms of hardness, stiffness, temperature resistance, and chemical stability. Polymers, however, are much lighter, cheaper, offer better impact resistance (toughness), and are extremely easy to manufacture into complex shapes.
Making the Right Choice for Your Application
The decision to use a ceramic, metal, or polymer should be driven by the single most critical requirement of your project.
- If your primary focus is extreme temperature or corrosive environments: Technical ceramics are often the only viable choice, offering stability where metals would rapidly degrade.
- If your primary focus is wear and abrasion resistance: The exceptional hardness of ceramics provides a service life that can far exceed that of even the hardest steels in abrasive conditions.
- If your primary focus is withstanding impacts and preventing catastrophic failure: A metal alloy is the superior choice, as it can absorb energy and deform safely.
- If your primary focus is low cost and ease of mass production: Polymers are typically the most cost-effective and simplest materials to shape and process for less demanding applications.
Ultimately, selecting a ceramic is a strategic engineering decision to gain unparalleled performance in environments that push all other materials past their limits.
Summary Table:
| Key Advantage | Why It Matters | Common Applications |
|---|---|---|
| Extreme Hardness & Wear Resistance | Resists abrasion and lasts longer than metals | Cutting tools, seals, liners |
| High-Temperature Stability | Maintains strength and shape above 1000°C | Furnace components, heat shields, turbines |
| Chemical & Corrosion Resistance | Inert to acids, alkalis, and oxidation | Chemical processing equipment, biomedical implants |
| Electrical & Thermal Insulation | Isolates high voltage and insulates against heat | Spark plugs, furnace linings, power line insulators |
Need high-performance materials for your lab or industrial process? KINTEK specializes in advanced lab equipment and consumables, including technical ceramics designed for extreme conditions. Our solutions ensure durability, precision, and reliability—whether you're handling corrosive chemicals, high temperatures, or abrasive environments. Contact us today to find the perfect ceramic component for your application!
Visual Guide
Related Products
- Silicon Carbide (SIC) Ceramic Sheet Wear-Resistant Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Alumina Al2O3 Ceramic Rod Insulated for Industrial Applications
- Zirconia Ceramic Gasket Insulating Engineering Advanced Fine Ceramics
- Precision Machined Zirconia Ceramic Ball for Engineering Advanced Fine Ceramics
- Hexagonal Boron Nitride HBN Ceramic Ring
People Also Ask
- Which is harder silicon carbide or tungsten carbide? Discover the Key to Material Selection
- What is the thermal expansion of SiC? Master Its Low CTE for Superior High-Temp Performance
- What is the resistivity of silicon carbide? It's a tunable property from <0.1 ohm-cm to highly resistive.
- What is the temperature resistance of silicon carbide? Withstands Extreme Heat Up to 1500°C
- What are the properties and applications of silicon carbide ceramics? Solve Extreme Engineering Challenges



















