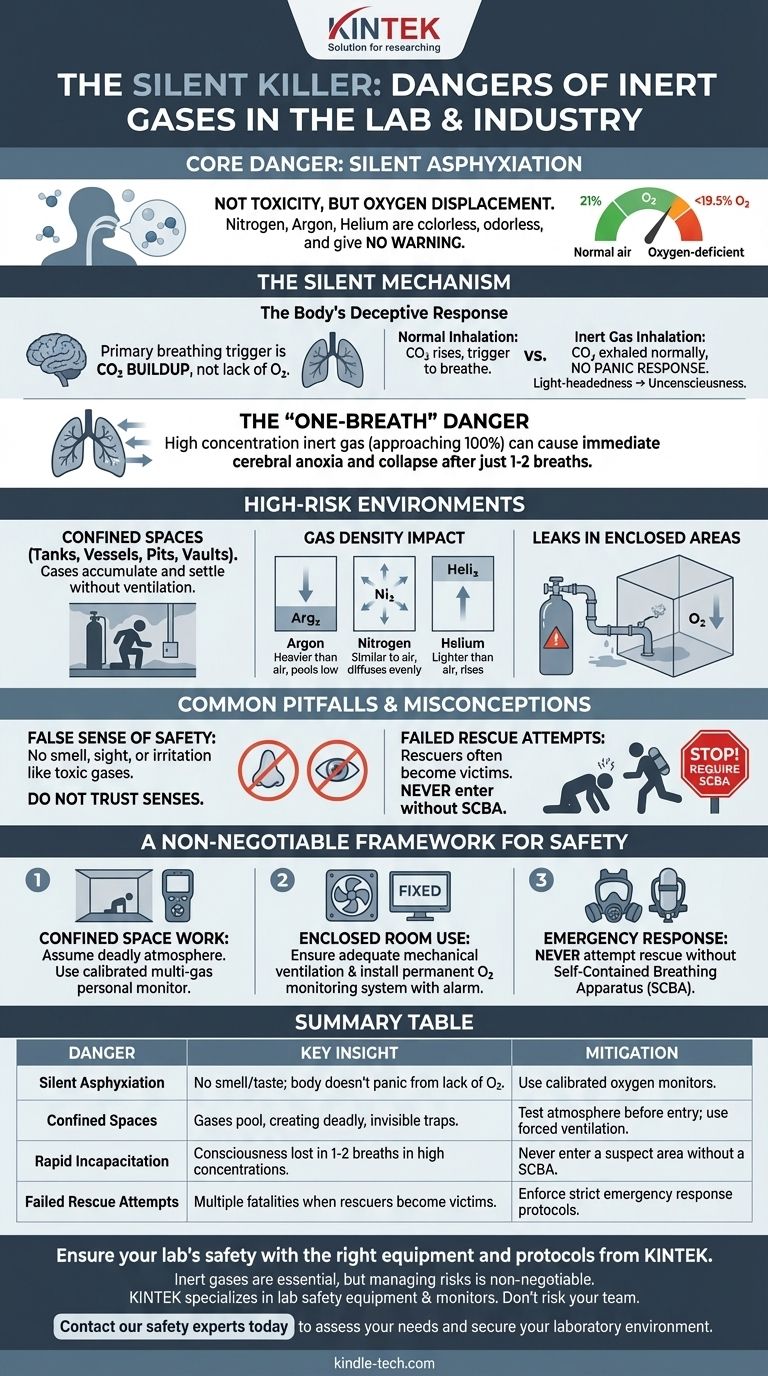
The primary danger of inert gases is not that they are toxic, but that they are silent and efficient asphyxiants. Because gases like nitrogen, argon, and helium are colorless and odorless, they can displace the breathable oxygen in an environment without providing any warning signals. A person can lose consciousness within seconds from breathing an oxygen-deficient atmosphere, often without any of the typical sensations of suffocation.
The greatest misconception about inert gases is equating "non-toxic" with "safe." The danger lies in their ability to silently displace oxygen, tricking the body's respiratory drive and leading to rapid incapacitation and death before a victim even realizes there is a problem.

The Silent Mechanism of Asphyxiation
Understanding how inert gases pose a threat requires a shift in thinking from poisoning to displacement. They don't attack the body; they simply remove a critical element required for life.
How Inert Gases Displace Oxygen
Normal air contains approximately 21% oxygen. When an inert gas is released into an enclosed or semi-enclosed space, it physically displaces the air, lowering the oxygen concentration.
Safety standards, such as those from OSHA, define an atmosphere with less than 19.5% oxygen as oxygen-deficient.
The Body's Deceptive Response
The primary trigger for the human body to breathe is the buildup of carbon dioxide (CO2) in the bloodstream, not the lack of oxygen.
When you inhale an inert gas, you continue to exhale CO2 normally. Since the CO2 levels don't rise abnormally, your body does not trigger the panic and air-hunger response associated with suffocation. You simply feel light-headed or dizzy before suddenly losing consciousness.
The "One-Breath" Danger
In an environment with a very high concentration of inert gas (approaching 100%), the effect is almost instantaneous.
Taking just one or two breaths can cause the oxygen in your lungs and bloodstream to be expelled, leading to immediate cerebral anoxia and collapse.
Identifying High-Risk Environments
The risk of asphyxiation is not uniform. The danger is magnified significantly in areas where gases can accumulate.
The Confined Space Threat
Confined spaces are the most common sites for inert gas fatalities. This includes tanks, vessels, utility vaults, pits, and any poorly ventilated room.
Without forced ventilation, inert gases can settle and remain for long periods, creating an invisible and deadly trap.
The Impact of Gas Density
The physical properties of the gas determine how it will behave in a room.
Gases heavier than air, like argon, will sink and pool in low-lying areas. Lighter gases, like helium, will rise. Nitrogen has a density very similar to air and will diffuse readily, filling a space more evenly. All are equally dangerous.
Leaks in Enclosed Areas
Even a small, slow leak from a compressed gas cylinder or a faulty pipe fitting can be fatal. Over hours, it can gradually lower the oxygen level in a storage closet, lab, or small room to a lethal concentration.
Common Pitfalls and Misconceptions
Trusting your senses is a fatal mistake when working with inert gases. The lack of warning signs leads to critical errors in judgment.
The False Sense of Safety
We are conditioned to recognize danger through smell, sight, or irritation. A toxic gas like ammonia or chlorine provides an immediate, aggressive warning to flee.
Inert gases offer no such warning. This absence of sensory input creates a powerful and dangerous illusion of safety.
Why Standard Safety Instincts Fail
You cannot "hold your breath" or "tough it out" against an oxygen-deficient atmosphere. The danger is not about willpower; it is about physiology. You will simply pass out.
The Hazard of Well-Intentioned Rescues
A tragic and common pattern in inert gas incidents involves multiple fatalities. A coworker sees a colleague collapse and rushes in to help, only to become a second victim of the same invisible hazard.
A rescuer must never enter a suspected oxygen-deficient atmosphere without a proper self-contained breathing apparatus (SCBA).
A Non-Negotiable Framework for Safety
Mitigating the risks of inert gases requires abandoning reliance on human senses and adopting a strict, proactive safety protocol.
- If your primary focus is working in a confined space: You must assume the atmosphere is deadly until proven otherwise with a properly calibrated, multi-gas personal monitor that alerts you to low oxygen levels.
- If your primary focus is using inert gases in any enclosed room: You must ensure adequate mechanical ventilation and consider installing a permanent oxygen monitoring system with an audible alarm.
- If your primary focus is emergency response: You must never attempt a rescue without the correct personal protective equipment, specifically a self-contained breathing apparatus (SCBA).
Ultimately, safety with inert gases is achieved not by reacting to danger, but by rigorously controlling the environment to prevent it from ever occurring.
Summary Table:
| Danger | Key Insight | Mitigation |
|---|---|---|
| Silent Asphyxiation | No smell or taste; body doesn't panic from lack of O2. | Use calibrated oxygen monitors. |
| Confined Spaces | Gases pool, creating deadly, invisible traps. | Test atmosphere before entry; use forced ventilation. |
| Rapid Incapacitation | Consciousness can be lost in 1-2 breaths in high concentrations. | Never enter a suspect area without a Self-Contained Breathing Apparatus (SCBA). |
| Failed Rescue Attempts | Multiple fatalities occur when rescuers become victims. | Enforce strict emergency response protocols. |
Ensure your lab's safety with the right equipment and protocols from KINTEK.
Inert gases are essential for many laboratory processes, but managing their risks is non-negotiable. KINTEK specializes in the lab equipment and consumables you need to work safely, from gas handling systems to environmental monitors. Don't let a false sense of security put your team at risk.
Contact our safety experts today to assess your needs and secure your laboratory environment.
Visual Guide
Related Products
- Hexagonal Boron Nitride HBN Ceramic Ring
- Hydraulic Diaphragm Lab Filter Press for Laboratory Filtration
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What is the purpose of applying a boron nitride (BN) coating to graphite molds? Enhance Sintering Release & Precision
- What is the function of a BN inner liner in a graphite mold during Flash Sintering? Master Precise Current Control
- What role does a Boron Nitride (BN) sleeve play in cold sintering mold assemblies? Essential Electrical Insulation
- Why is it necessary to use high-temperature and corrosion-resistant ceramics for H2SO4 decomposers in the IS process?
- How do alumina ceramic tubes address technical challenges in electrochemical devices? Ensure Peak Signal Integrity.



















