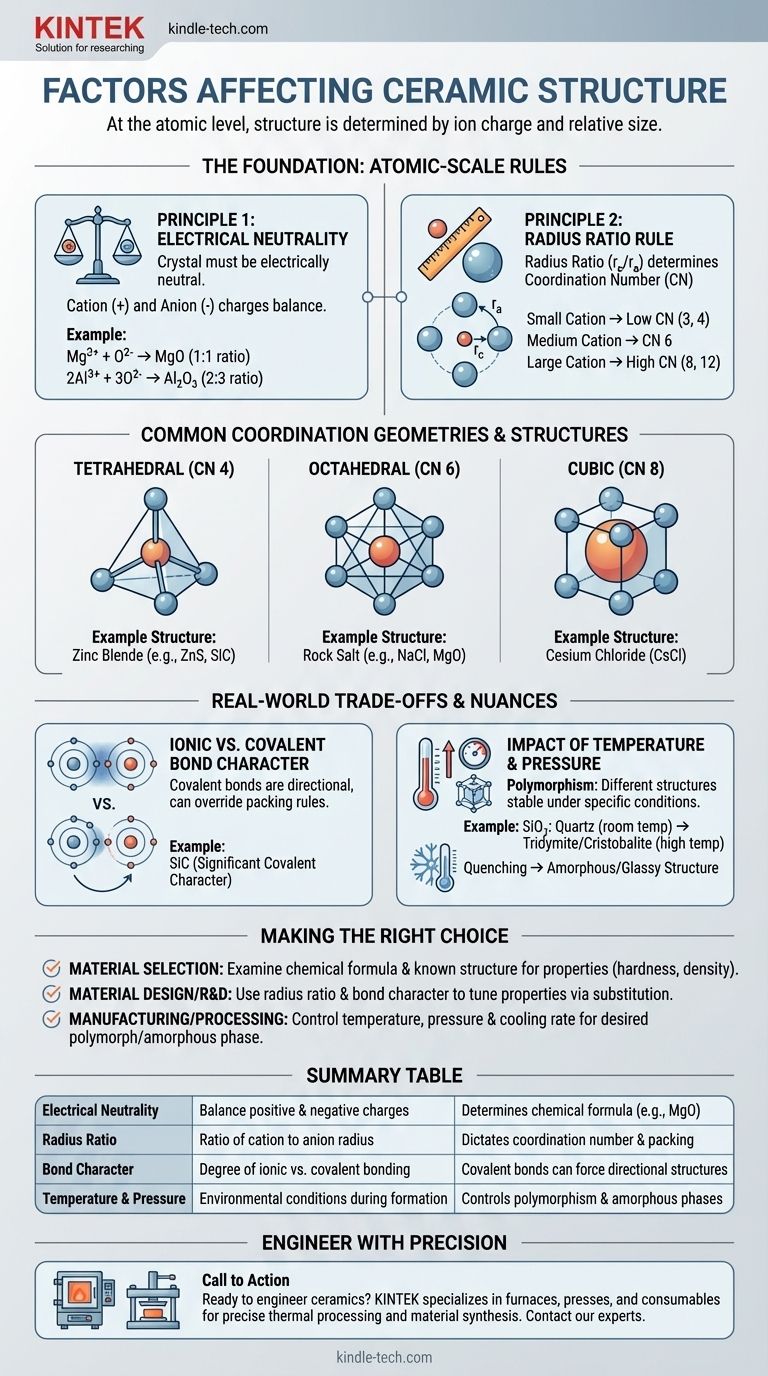
At the atomic level, a ceramic's crystal structure is determined by two primary factors: the electrical charge of its constituent ions and the relative size of those ions. To form a stable, low-energy structure, the arrangement of atoms must satisfy charge neutrality while also packing together in the most geometrically efficient way possible.
The fundamental challenge in forming any ceramic crystal structure is balancing competing forces. Cations and anions attract, creating dense structures, but their relative sizes dictate the specific geometric arrangement—the coordination number—that can be physically achieved while maintaining overall electrical neutrality.
The Foundation: Atomic-Scale Rules
To understand why a ceramic like magnesium oxide (MgO) forms one structure while silicon carbide (SiC) forms another, we must start with the two non-negotiable rules that govern how atoms arrange themselves into a stable crystal lattice.
Principle 1: The Rule of Electrical Neutrality
The most fundamental requirement is that the crystal must be electrically neutral. The sum of all positive charges from the cations must be precisely balanced by the sum of all negative charges from the anions.
This principle dictates the chemical formula itself. For example, since a magnesium ion has a +2 charge (Mg²⁺) and an oxygen ion has a -2 charge (O²⁻), they combine in a 1:1 ratio to form MgO. An aluminum ion (Al³⁺) and an oxygen ion (O²⁻) must combine in a 2:3 ratio to form Al₂O₃ to achieve neutrality.
Principle 2: The Radius Ratio Rule
Once the chemical formula is set, geometry takes over. The radius ratio—the radius of the cation (r_c) divided by the radius of the anion (r_a)—is the critical factor that determines the coordination number (CN).
The coordination number is simply the number of anions that can pack around a central cation. Imagine trying to pack basketballs (anions) around a smaller tennis ball (cation). You can only fit a few before they start touching each other. If you replace the tennis ball with a larger soccer ball, you can fit more basketballs around it. The relative size dictates the packing geometry.
From Atomic Packing to Crystal Structure
These two principles work together to produce the repeating, three-dimensional patterns that define ceramic crystal structures. The radius ratio predicts the coordination number, and the need for charge neutrality then dictates how these coordinated units link together in space.
Common Coordination Geometries
Specific radius ratio ranges strongly suggest a preferred coordination number and its corresponding shape:
- A small cation results in a low CN, like 3 (triangular) or 4 (tetrahedral).
- A medium-sized cation allows for a CN of 6 (octahedral).
- A large cation, nearly the size of the anion, can achieve a CN of 8 (cubic) or even 12.
Examples of Common Ceramic Structures
These rules give rise to well-known crystal structures named after common minerals. For simple AX-type ceramics (one cation, one anion):
- Rock Salt Structure (e.g., NaCl, MgO): Features a coordination number of 6 for both the cation and anion. It is a highly stable, common structure when the cation and anion have a moderate size difference.
- Cesium Chloride Structure (CsCl): Occurs when the cation is almost as large as the anion, allowing for a more tightly packed coordination number of 8.
- Zinc Blende Structure (e.g., ZnS, SiC): Forms when the cation is significantly smaller than the anion, resulting in a coordination number of 4 (tetrahedral). This structure is also characteristic of materials with strong covalent bonding.
For more complex formulas like AₘXₚ (e.g., Al₂O₃, CaF₂), the same principles apply. The structure simply becomes a more intricate arrangement to ensure every ion achieves its preferred coordination and the overall charge remains neutral.
Understanding the Trade-offs and Limitations
While these principles provide a powerful framework, they are a simplified model. Real-world factors introduce important nuances that can alter the final structure.
The Ionic vs. Covalent Bond Character
The radius ratio rule works best for purely ionic bonds. However, many ceramics, like silicon carbide (SiC) and silicon nitride (Si₃N₄), have significant covalent character.
Covalent bonds are highly directional. Atoms prefer to bond at specific angles (e.g., 109.5° in a tetrahedron). In these materials, the need to satisfy directional covalent bonds can override the geometric packing rules of the radius ratio, forcing a specific structure like tetrahedral coordination.
The Impact of Temperature and Pressure
A single chemical compound can often exist in multiple different crystal structures, a phenomenon known as polymorphism. Each of these structures, or polymorphs, is stable under a specific range of temperature and pressure.
For example, silica (SiO₂) exists as quartz at room temperature, but transforms into other polymorphs like tridymite and cristobalite at higher temperatures. These transformations involve a rearrangement of the atoms into a new, more stable structure for those conditions.
Furthermore, if a molten ceramic is cooled very rapidly (quenching), the atoms may not have enough time to arrange themselves into an ordered crystal lattice. This results in a disordered, amorphous, or glassy structure.
Making the Right Choice for Your Application
Understanding these factors allows you to connect a ceramic's processing and composition to its final structure and, ultimately, its performance.
- If your primary focus is material selection: Begin by examining the chemical formula and known crystal structure. A dense, high-coordination structure like corundum (Al₂O₃) implies high hardness and density, while a lower-coordination structure may have different properties.
- If your primary focus is material design or R&D: Use the radius ratio and bond character as your tools. Substituting atoms with different sizes or electronegativity can be used to intentionally shift the crystal structure and tune its properties.
- If your primary focus is manufacturing and processing: Your key variables are temperature, pressure, and cooling rate. Use them to control which polymorph forms or to decide between a crystalline and an amorphous final product.
By grasping the interplay of charge, size, and processing conditions, you can move from simply using ceramics to deliberately engineering them for a specific purpose.
Summary Table:
| Factor | Description | Key Impact on Structure |
|---|---|---|
| Electrical Neutrality | The total positive and negative charges in the crystal must balance. | Determines the chemical formula (e.g., MgO, Al₂O₃). |
| Radius Ratio | The ratio of the cation radius to the anion radius (r_c/r_a). | Dictates the coordination number and packing geometry (e.g., tetrahedral, octahedral). |
| Bond Character | The degree of ionic vs. covalent bonding. | Covalent bonds can force directional structures, overriding simple packing rules. |
| Temperature & Pressure | The environmental conditions during formation and processing. | Controls polymorphism (different crystal forms) and the formation of amorphous/glassy phases. |
Ready to engineer ceramics with precision? The right lab equipment is critical for controlling the factors that define your material's structure and performance. KINTEK specializes in the furnaces, presses, and consumables your laboratory needs for precise thermal processing and material synthesis. Contact our experts today to discuss how our solutions can help you achieve your specific material goals.
Visual Guide
Related Products
- Silicon Carbide (SIC) Ceramic Sheet Wear-Resistant Engineering Advanced Fine Ceramics
- Precision Machined Silicon Nitride (SiN) Ceramic Sheet for Engineering Advanced Fine Ceramics
- Laboratory Test Sieves and Sieving Machines
- Precision Machined Zirconia Ceramic Ball for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Aluminum Oxide Al2O3 Heat Sink for Insulation
People Also Ask
- What is the main disadvantage of ceramics? The Critical Challenge of Brittleness
- What is better than Rockwool insulation? Maximize Thermal or Fire Performance
- How is sintering done to ceramics? Master the Process for High-Performance Materials
- What is the process of hot isostatic pressing for making ceramic matrix composites? Achieve Near-Zero Porosity for Superior Performance
- What is the driving force for sintering a ceramic? Harnessing Energy Reduction for Stronger Materials
- What is meant by ceramic powder? The Engineered Blueprint for Advanced Ceramics
- What are the characteristics of SiC? Unlock High-Temp, Hard, and Chemically Inert Performance
- Why is it necessary to use sintering aids for PLS? Achieve Full Density in Ultra-High Temperature Ceramics



















