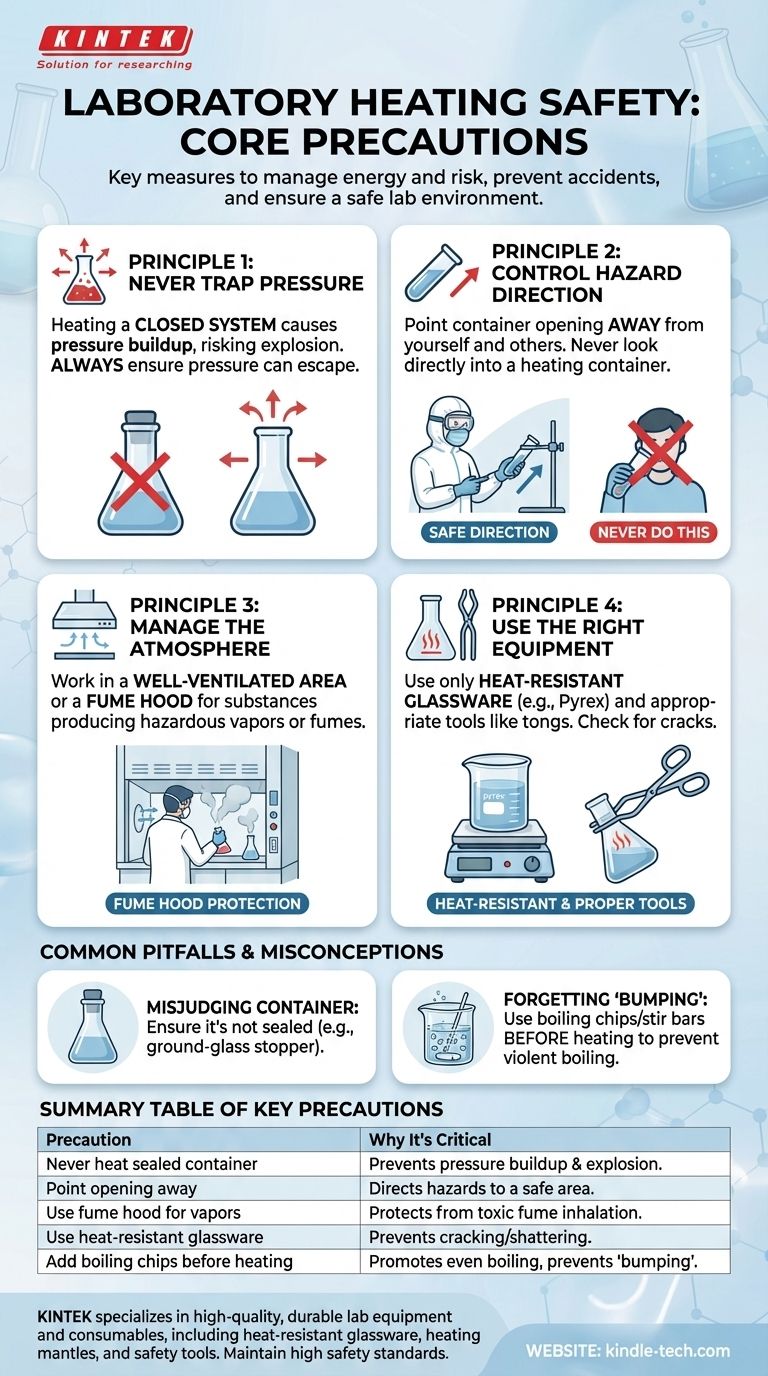
When heating any substance in a laboratory, your safety and the safety of those around you depend on a strict set of protocols. The most critical precautions are to never heat a fully sealed container, to always point the opening of the container away from yourself and others, and to use a fume hood when working with substances that may produce hazardous vapors.
The core principle behind all heating precautions is managing energy and risk. Heating introduces energy that can cause sudden, violent changes like pressure buildup, splashing, or toxic fume release. Your primary goal is to control this energy by ensuring pressure can escape safely, directing any potential hazard away from people, and containing all fumes.

The Core Principles of Safe Heating
Understanding why these rules exist is the key to applying them correctly in any situation. The precautions are not arbitrary; they are based on the fundamental physics and chemistry of heating materials.
Principle 1: Never Trap Pressure
When you heat a substance, especially a liquid, it expands or turns into a gas. This conversion dramatically increases pressure inside the container.
Heating a closed system prevents this pressure from escaping. The container effectively becomes a small bomb, which can shatter and project hot, hazardous materials and glass shards across the lab.
Principle 2: Control the Direction of Hazard
The open end of a test tube or flask is the primary escape route for pressure, vapor, and potential splashes.
Never look directly into the container you are heating. A sudden boiling event, known as "bumping," can eject the contents straight up into your face.
Likewise, never point the opening of the container toward anyone. Treat it like the muzzle of a weapon; always ensure it is aimed at a safe, unoccupied area, typically towards the back of a fume hood or a wall.
Principle 3: Manage the Atmosphere
Heating can release vapors. Even if a substance is not overtly toxic, its fumes can be irritating or flammable.
Always work in a well-ventilated area. If there is any chance the substance will produce toxic, corrosive, or noxious fumes, it is mandatory to perform the entire procedure inside a laboratory fume hood. This protects you from inhaling dangerous chemicals.
Principle 4: Use the Right Equipment
Standard equipment is not designed to handle thermal stress. Using the wrong gear is a common and preventable cause of accidents.
Only use heat-resistant glassware, such as Pyrex or Kimax (borosilicate glass). Standard glass will crack or shatter when heated. Also, inspect this glassware for any small cracks or chips before heating, as these weaknesses can cause it to fail.
Always use appropriate tools like tongs or clamps to handle hot containers. Never handle them with your hands, even with gloves.
Common Pitfalls and Misconceptions
Vigilance is essential because it's easy to become complacent. Several common mistakes can lead to serious injury.
Misjudging the Container
The most dangerous mistake is heating a container that appears open but is actually sealed. This often happens with flasks fitted with a ground-glass stopper that has not been removed or vented.
Forgetting About "Bumping"
Liquids do not always boil smoothly. They can superheat and then boil violently all at once in an event called "bumping." This ejects the hot liquid from the container.
To prevent this, always add boiling chips or a magnetic stir bar to the liquid before you begin heating. Never add them to an already hot liquid, as this can trigger an immediate and violent boiling event.
Ignoring Personal Protective Equipment (PPE)
Your PPE is your last line of defense. Safety goggles, a lab coat, and proper gloves are non-negotiable. Goggles protect you from splashes, and a lab coat protects your skin and clothing from spills.
Making the Right Choice for Your Task
Adapt your precautions based on the specific substance and procedure you are using.
- If your primary focus is heating a small amount of liquid in a test tube: Your priority is preventing bumping and directing splashes. Use boiling chips and angle the test tube at 45 degrees, pointing away from everyone.
- If your primary focus is boiling a volatile or flammable solvent: Your priority is ventilation and fire prevention. You must use a fume hood and a flameless heating source like a heating mantle or steam bath.
- If your primary focus is reacting chemicals that produce fumes: Your priority is containing all vapors. Perform the entire experiment in a certified fume hood with the sash at the proper height.
A deliberate and safety-conscious approach is the hallmark of a true professional in the laboratory.
Summary Table:
| Key Precaution | Why It's Critical |
|---|---|
| Never heat a sealed container | Prevents pressure buildup and explosion from gas expansion. |
| Point container opening away | Directs potential splashes, vapors, and pressure release to a safe area. |
| Use a fume hood for hazardous vapors | Protects from inhaling toxic, corrosive, or flammable fumes. |
| Use heat-resistant glassware (e.g., Pyrex) | Prevents cracking or shattering under thermal stress. |
| Add boiling chips/stir bar before heating | Promotes even boiling and prevents violent "bumping." |
Ensure your lab's heating procedures are safe and efficient with the right equipment. KINTEK specializes in high-quality, durable lab equipment and consumables, including heat-resistant glassware, heating mantles, and safety tools designed for precise and secure operations. Our products help you maintain the highest safety standards while achieving accurate results.
Contact us today to find the perfect heating solutions for your laboratory's specific needs and enhance your lab's safety protocol. Get in touch with our experts now!
Visual Guide
Related Products
- 20L Heating Chilling Circulator Cooling Water Bath Circulator for High and Low Temperature Constant Temperature Reaction
- 50L Heating Chilling Circulator Cooling Water Bath Circulator for High and Low Temperature Constant Temperature Reaction
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- 30L Heating Chilling Circulator Cooling Water Bath Circulator for High and Low Temperature Constant Temperature Reaction
- 10L Heating Chilling Circulator Cooling Water Bath Circulator for High and Low Temperature Constant Temperature Reaction
People Also Ask
- What role does a high-precision constant temperature circulating water bath play in AEM research? Stability & Control
- What are water baths used for? Achieve Precise & Gentle Temperature Control for Your Lab Samples
- How do circulating cooling systems ensure accuracy in adsorption tests? Stabilize Thermal Variables for Precise Data
- What is the primary function of water baths and chillers? Mastering Precise Thermal Stability for Liquid Samples
- How does a water bath work? Master Precise and Gentle Heating for Your Lab



















