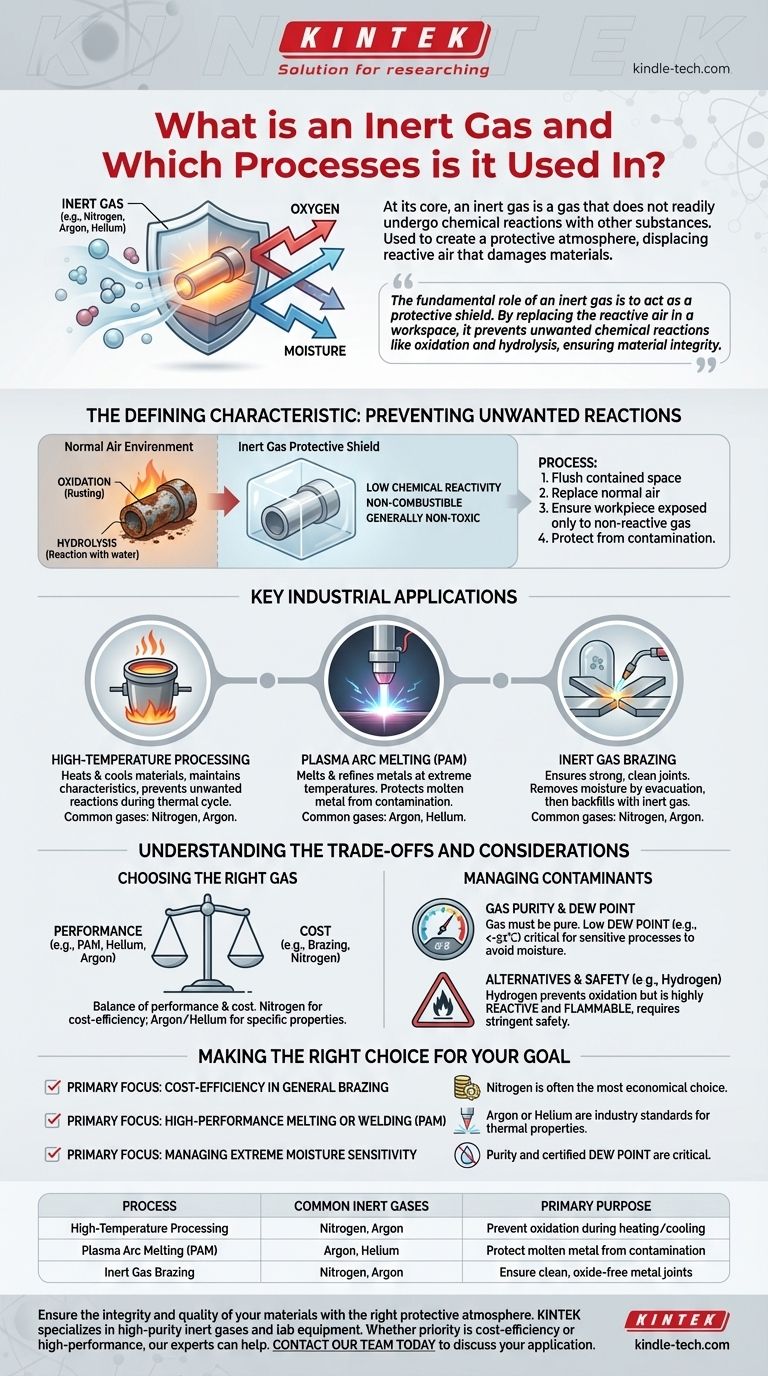
At its core, an inert gas is a gas that does not readily undergo chemical reactions with other substances. This non-reactive quality is their defining characteristic and primary value. They are used in industrial processes to create a protective atmosphere, displacing the reactive air (primarily oxygen and moisture) that would otherwise damage or contaminate the materials being worked on. Common examples include nitrogen, argon, and helium.
The fundamental role of an inert gas is to act as a protective shield. By replacing the reactive air in a workspace, it prevents unwanted chemical reactions like oxidation (rusting) and hydrolysis (reaction with water), ensuring the integrity of materials during sensitive manufacturing processes.

The Defining Characteristic: Preventing Unwanted Reactions
The value of inert gases comes from what they don't do. In an environment filled with oxygen and water vapor, many materials, especially when heated, will react in undesirable ways. Inert gases provide a solution by creating a controlled, non-reactive environment.
What Makes a Gas "Inert"?
Inert gases have very low chemical reactivity. This stability means they are non-combustible and generally non-toxic. This property allows them to be introduced into highly sensitive processes without interfering with the desired chemical or physical changes.
The Primary Threats: Oxidation and Hydrolysis
The two most common unwanted reactions are with components in the ambient air. Oxidation occurs when a material reacts with oxygen, while hydrolysis is a reaction with water or moisture. Both can degrade the quality, strength, and purity of a final product.
Creating a Protective Shield
The practical application involves using the inert gas to flush out and replace the normal air in a contained space, such as a chamber or around a welding torch. This displacement ensures that the workpiece is only exposed to the non-reactive gas, protecting it from contamination.
Key Industrial Applications
The need to prevent contamination is critical in many high-precision and high-temperature manufacturing processes. Inert gases are essential for achieving the required quality and performance in these fields.
High-Temperature Processing
Many materials become significantly more reactive at high temperatures. Inert gases are used to heat and cool materials while ensuring they maintain their specific characteristics, preventing unwanted reactions that would occur in normal air during the thermal cycle.
Plasma Arc Melting (PAM)
Plasma Arc Melting is a process that uses extremely high temperatures to melt and refine metals. To protect the molten metal from contamination, a controlled inert atmosphere is essential. The most common inert gases used for this application are Helium or Argon.
Inert Gas Brazing
Brazing is a process used to join two pieces of metal. To ensure a strong, clean joint, the metal surfaces must be free from oxides. Brazing is often performed in sealed chambers where heating and evacuation are first used to remove moisture before the chamber is backfilled with an inert gas.
Understanding the Trade-offs and Considerations
While the principle is simple, the choice and management of an inert gas depend on the specific application, cost, and required purity level.
Choosing the Right Gas
The selection of an inert gas is a balance of performance and cost. For example, in brazing applications, Nitrogen is a very common choice because it is effective and significantly less expensive than other options. For more demanding processes like PAM, the specific properties of Argon or Helium are required.
Managing Contaminants in the Gas
The inert gas itself must be pure. For sensitive processes like brazing, the gas must have a very low dew point (a measure of moisture content), often specified to be less than -51℃. This ensures the protective atmosphere isn't inadvertently introducing the very contaminant it is meant to eliminate.
Alternatives and Safety
In some cases, other gases are used to achieve a similar protective effect. Hydrogen, for instance, is excellent at preventing oxidation. However, unlike a true inert gas, hydrogen is highly reactive and flammable, making it a more dangerous alternative that requires stringent safety protocols.
Making the Right Choice for Your Goal
Selecting the appropriate gas and process parameters is critical for ensuring product quality and operational efficiency. The decision should always be tied directly to the specific requirements of the material and the process.
- If your primary focus is cost-efficiency in general brazing: Nitrogen is often the most economical choice for creating a basic, protective atmosphere.
- If your primary focus is high-performance melting or welding (like PAM): Argon or Helium are the industry standards, chosen for their specific plasma and thermal properties.
- If your primary focus is managing extreme moisture sensitivity: The purity and certified dew point of your inert gas supply are as critical as the choice of gas itself.
Ultimately, using an inert gas correctly is a foundational step in guaranteeing the quality, strength, and integrity of your final product.
Summary Table:
| Process | Common Inert Gases Used | Primary Purpose |
|---|---|---|
| High-Temperature Processing | Nitrogen, Argon | Prevent oxidation during heating/cooling |
| Plasma Arc Melting (PAM) | Argon, Helium | Protect molten metal from contamination |
| Inert Gas Brazing | Nitrogen, Argon | Ensure clean, oxide-free metal joints |
Ensure the integrity and quality of your materials with the right protective atmosphere. KINTEK specializes in providing high-purity inert gases and lab equipment for all your industrial and laboratory needs. Whether your priority is cost-efficiency with nitrogen or high-performance with argon, our experts can help you select the optimal solution. Contact our team today to discuss your specific application and guarantee the strength and purity of your final product.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- How to make an inert atmosphere? A Step-by-Step Guide for Protecting Sensitive Materials
- How does an oxygen or carbon probe measure carbon potential? The Science Behind Precise Furnace Control
- How does an atmosphere control system influence wood-plastic composites? Master Thermal Stability and Material Safety
- What is controlled atmosphere in heat treatment? Master Surface Chemistry for Superior Metal Parts
- What are the safety precautions for argon welding? Essential Guide to Protecting Against UV, Fumes, Shock, and Asphyxiation
- What is the role of a 700°C oxygen atmosphere furnace in LiCoO2 cathode preparation? Unlock High-Performance Batteries
- What type of gases is used in a heat treat furnace? Control Your Metal's Final Properties
- What are the benefits of using an atmosphere-controlled furnace for 316LN annealing? Preserve Strength and Surface.



















