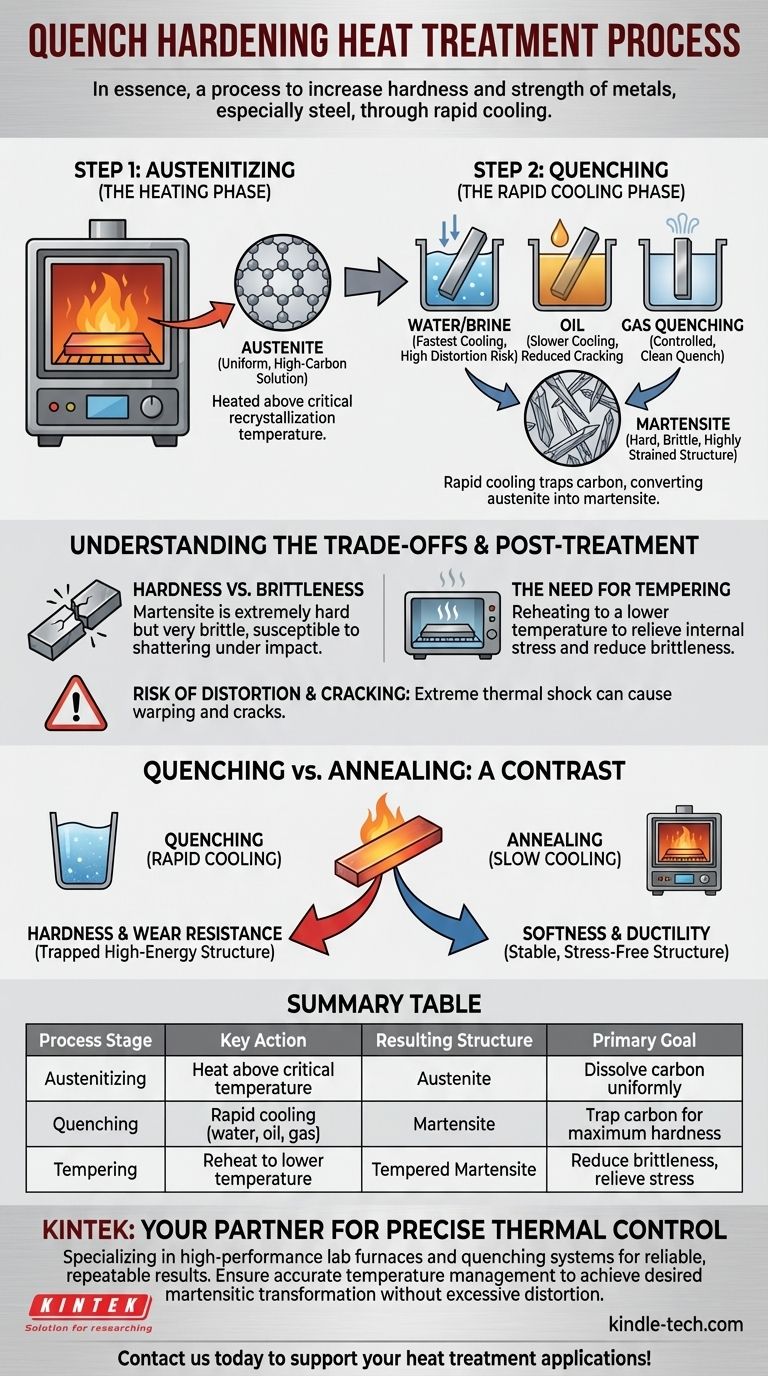
In essence, quench hardening is a heat treatment process used to increase the hardness and strength of metals, particularly steel. The process involves heating the metal to a specific high temperature and then cooling it with extreme rapidity by immersing it in a medium like water, oil, or gas. This rapid cooling locks the metal's internal crystal structure into a hard, stressed state.
Quenching is not merely about cooling a hot piece of metal. It is a precisely controlled process of cooling at a rate fast enough to prevent the formation of softer, more stable crystal structures, thereby trapping the material in a hard, meta-stable state known as martensite.

The Science of Quenching: A Two-Step Process
True quench hardening is a combination of two distinct thermal stages: austenitizing (heating) and quenching (rapid cooling). Understanding both is key to understanding the final result.
Step 1: Austenitizing (The Heating Phase)
Before a metal can be hardened, it must first be properly heated. This stage transforms its internal structure into a uniform, high-energy state.
The metal, typically a carbon steel, is heated above its critical recrystallization temperature. At this point, the existing crystal structure dissolves into a new phase called austenite.
In the austenitic state, carbon atoms are evenly dissolved within the iron crystal lattice. This uniform, high-carbon solution is the necessary starting point for creating a hardened structure.
Step 2: Quenching (The Rapid Cooling Phase)
The quench is what traps the high-energy state created during heating, converting it into hardness.
The goal of the quench is to cool the metal so quickly that the dissolved carbon atoms do not have time to move and form softer, more stable structures like pearlite or bainite.
This rapid cooling forces the austenite to transform into martensite, a very hard, brittle, and highly strained body-centered tetragonal crystal structure. This transformation is the source of the hardness gained in the process.
There are several methods for achieving this rapid cooling, each with a different cooling rate and severity:
- Water/Brine: Provides the fastest cooling rate, but creates the highest risk of distortion and cracking.
- Oil: Cools slower than water, reducing cracking risk while still achieving significant hardness.
- Gas Quenching: Modern vacuum furnaces use high-pressure gases like nitrogen or argon for a controlled, clean quench, often for high-value components.
Understanding the Trade-offs
Quench hardening produces exceptional hardness, but this property does not come without significant compromises that must be managed.
Hardness vs. Brittleness
The primary trade-off is that the martensitic structure, while extremely hard, is also very brittle. A fully quench-hardened part is often too brittle for most practical applications as it can shatter under impact.
The Need for Tempering
Because of this brittleness, a quenched part is almost always followed by a secondary heat treatment called tempering. Tempering involves reheating the part to a lower temperature to relieve some internal stress and reduce brittleness, albeit with a slight reduction in overall hardness.
Risk of Distortion and Cracking
The extreme thermal shock of plunging a red-hot part into a cool liquid creates massive internal stresses. This can cause the part to warp, distort, or even develop microscopic or catastrophic cracks during the process.
Quenching vs. Annealing: Hardness vs. Ductility
To fully grasp the purpose of quenching, it is useful to contrast it with its opposite: annealing.
Quenching for Hardness
Quenching is defined by rapid cooling. This process is designed to trap a disorganized, high-energy crystal structure (martensite) to maximize hardness and wear resistance.
Annealing for Softness
Annealing involves heating a metal and then cooling it very slowly, often by letting it cool inside the furnace. This slow cooling allows a stable, stress-free, and ductile (soft) grain structure to form, making the metal easier to machine or form.
Making the Right Choice for Your Goal
The correct heat treatment is entirely dependent on the desired final properties of the component.
- If your primary focus is maximum hardness and wear resistance: Use quench hardening, but plan for a subsequent tempering process to achieve a usable balance of hardness and toughness.
- If your primary focus is to soften metal for easier machining or forming: Use annealing to create a ductile, stress-relieved structure.
- If you are working with certain non-ferrous alloys: Be aware that quenching can sometimes produce a softer state, so you must verify the correct treatment for that specific material.
Ultimately, controlling the rate of cooling is the most powerful tool for dictating the final mechanical properties of a heat-treated metal.
Summary Table:
| Process Stage | Key Action | Resulting Structure | Primary Goal |
|---|---|---|---|
| Austenitizing | Heat above critical temperature | Austenite | Dissolve carbon uniformly |
| Quenching | Rapid cooling (water, oil, gas) | Martensite | Trap carbon for maximum hardness |
| Tempering | Reheat to lower temperature | Tempered Martensite | Reduce brittleness, relieve stress |
Need precise thermal control for your quench hardening processes? KINTEK specializes in high-performance lab furnaces and quenching systems designed for reliable, repeatable results. Our equipment ensures the accurate temperature management critical for achieving the desired martensitic transformation without excessive distortion or cracking. Whether you are developing new alloys or hardening components, KINTEK provides the robust solutions your laboratory needs. Contact us today to discuss how we can support your heat treatment applications!
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Brazing Furnace
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
People Also Ask
- What are the benefits of vacuum annealing? Achieve Pristine, Oxide-Free Parts with Superior Material Properties
- How do annular steam pipes improve activation furnace efficiency? Maximize Carbon Reaction Rates and Quality
- What is the principle of vacuum system? Creating a Controlled Low-Pressure Environment
- What are the safety precautions for heat treatment process? A Guide to Managing Thermal, Atmospheric, and Equipment Risks
- What is the temperature of the arc melting furnace? Achieve 3000°C for Refractory Metals
- Why is a vacuum drying oven necessary for treating WTaVTiZrx powder? Achieve High-Density, Defect-Free Laser Cladding
- How does a high-temperature laboratory furnace modify Li–Al LDH during catalyst pretreatment? Enhance Catalytic Activity
- What is the temperature range of an electric furnace? Achieve Safe, Efficient Home Heating



















