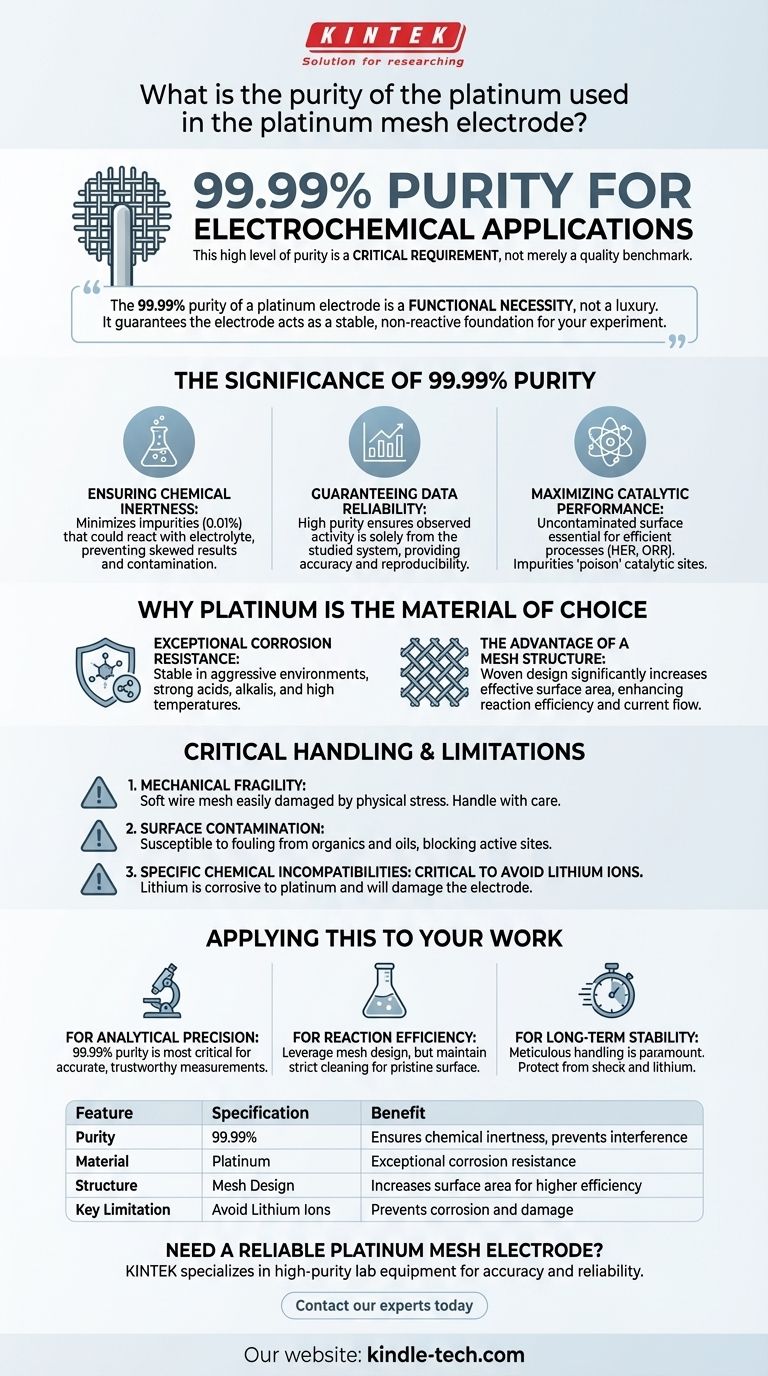
For electrochemical applications, the standard purity of the platinum used in a platinum mesh electrode is 99.99%. This high level of purity is not merely a quality benchmark but a critical requirement, as it ensures the electrode remains chemically inert and does not introduce variables that could compromise the accuracy and reliability of experimental data.
The 99.99% purity of a platinum electrode is a functional necessity, not a luxury. It guarantees the electrode acts as a stable, non-reactive foundation for your experiment, ensuring that the results you observe are due to your intended reaction, not interference from the measurement tool itself.

The Significance of 99.99% Purity
The choice of such high-purity material is a deliberate engineering decision rooted in the fundamental requirements of electrochemistry. Even the smallest impurities can have an outsized impact on sensitive measurements.
Ensuring Chemical Inertness
Impurities, which constitute the remaining 0.01%, could potentially react with the electrolyte or analyte. These unintended side reactions can skew results, generate false positives, or contaminate the entire system.
By using 99.99% pure platinum, you create a controlled environment where the electrode surface is as non-reactive as possible, providing a stable baseline.
Guaranteeing Data Reliability
The primary goal of an electrode is to facilitate a reaction or measurement without becoming part of it. The high purity of the platinum ensures that any observed electrochemical activity can be confidently attributed to the system being studied.
This provides a solid guarantee for the accuracy and reproducibility of your experimental data.
Maximizing Catalytic Performance
Platinum is an excellent catalyst for key electrochemical processes, such as the hydrogen evolution reaction (HER) and oxygen reduction reaction (ORR).
A pure, uncontaminated surface is essential for this catalytic activity. Impurities can "poison" the catalytic sites on the electrode surface, reducing reaction efficiency and altering the electrode's performance characteristics.
Why Platinum is the Material of Choice
Beyond purity, platinum possesses a unique combination of properties that make it an ideal material for high-performance electrodes. Its role is defined by its inherent stability and efficiency.
Exceptional Corrosion Resistance
Platinum is exceptionally stable in aggressive environments. It demonstrates remarkable resistance to corrosion from strong acids (like hydrochloric, sulfuric, and nitric acid), strong alkalis, and high temperatures.
This durability allows it to function reliably for long periods without being degraded by the chemicals in the electrolyte.
The Advantage of a Mesh Structure
A platinum mesh electrode is woven from high-purity platinum wire. This mesh design significantly increases the effective surface area compared to a solid sheet or wire of the same dimensions.
A larger surface area directly enhances the efficiency of electrochemical reactions by providing more sites for the reaction to occur, which is crucial for applications requiring high current flow or fast reaction rates.
Critical Handling and Limitations
While robust chemically, a high-purity platinum mesh electrode requires careful handling to maintain its integrity and performance. Understanding its limitations is key to its longevity.
Mechanical Fragility
The platinum wire used to create the mesh is soft and malleable. The electrode can be easily damaged by mechanical stress, such as being dropped, bent, or subjected to direct pressure. Always handle it with care.
Susceptibility to Surface Contamination
The performance of the electrode is highly dependent on the condition of its surface. Contact with organic substances, oils from your skin, or other materials can "foul" the electrode.
This fouling can block active sites, inhibit catalytic activity, and introduce contaminants into your experiment.
Specific Chemical Incompatibilities
Despite its broad resistance, platinum is not invincible. It is critical to avoid any contact with lithium ions, which are known to be corrosive to platinum. Using this electrode in lithium-based systems is prohibited and will damage it.
Applying This to Your Work
Your approach to using a platinum mesh electrode should be guided by your primary experimental goal.
- If your primary focus is analytical precision: The 99.99% purity is the most critical feature, as it minimizes interference and is the foundation for accurate, trustworthy measurements.
- If your primary focus is reaction efficiency: Leverage the mesh design for its high surface area, but maintain a strict cleaning protocol to ensure the catalytic surface remains pristine and active.
- If your primary focus is long-term stability: Meticulous handling is paramount. Protect the electrode from mechanical shock and chemical contaminants, especially lithium, to ensure its longevity.
Ultimately, treating your platinum electrode as a precision instrument is the key to unlocking reliable and repeatable results.
Summary Table:
| Feature | Specification | Benefit |
|---|---|---|
| Purity | 99.99% | Ensures chemical inertness and prevents experimental interference |
| Material | Platinum | Exceptional corrosion resistance in acids, alkalis, and high temperatures |
| Structure | Mesh Design | Increases surface area for higher reaction efficiency and current flow |
| Key Limitation | Avoid Lithium Ions | Prevents corrosion and damage to the electrode |
Need a reliable platinum mesh electrode for your lab? KINTEK specializes in high-purity lab equipment and consumables, ensuring your electrochemical experiments are built on a foundation of accuracy and reliability. Our 99.99% pure platinum electrodes are designed for researchers who demand uncompromised data quality and long-term stability. Contact our experts today to find the perfect electrode solution for your specific application!
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Gold Disc Electrode
- Metal Disc Electrode Electrochemical Electrode
People Also Ask
- What are the performance characteristics of platinum sheet electrodes? Unlock Superior Electrochemical Performance
- What precautions should be taken when using a platinum sheet electrode? Ensure Accurate & Reproducible Electrochemical Data
- What is the expected lifespan of a platinum sheet electrode? Maximize Your Electrode's Service Life
- What are the key performance characteristics and applications of platinum sheets? Unmatched Reliability for Demanding Applications
- What are the available specifications for platinum sheet electrodes? Find the Perfect Fit for Your Electrochemical Needs



















