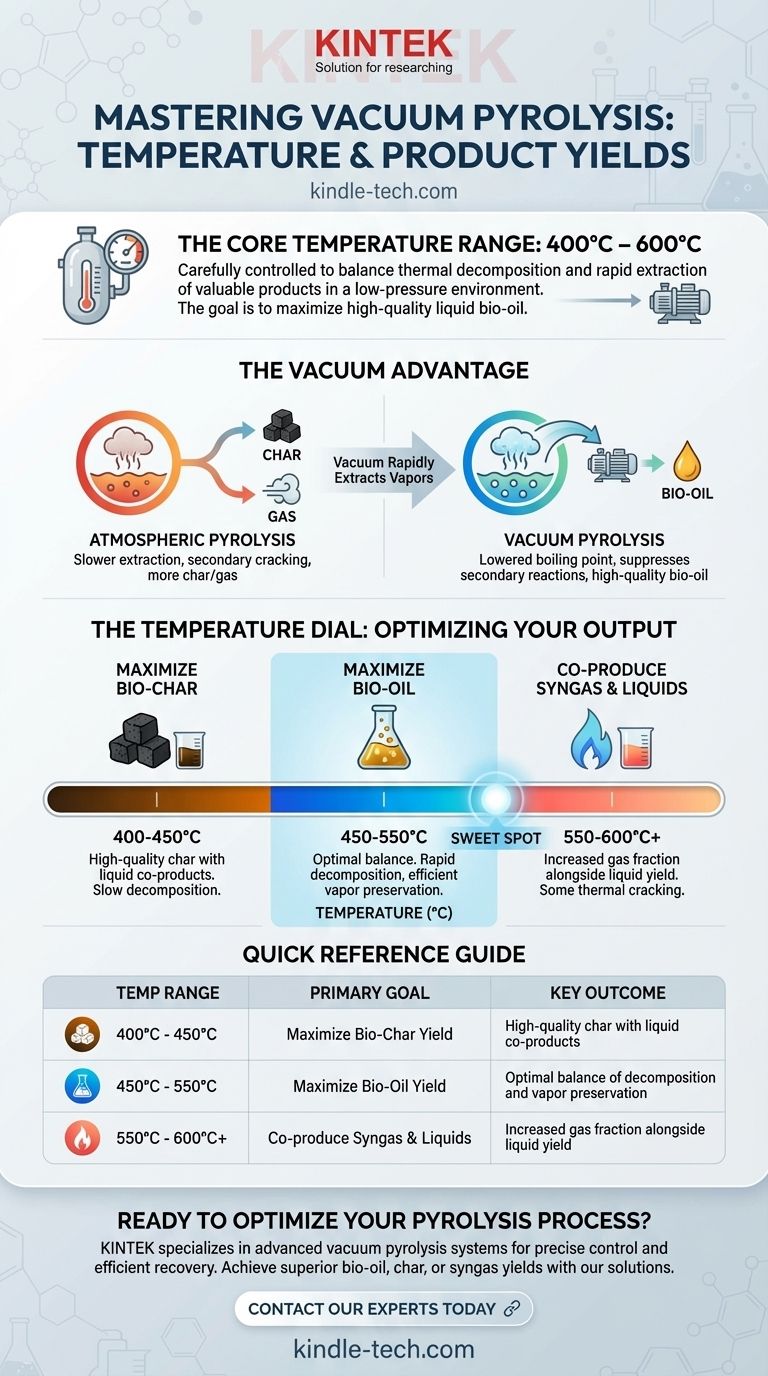
In vacuum pyrolysis, the operational temperature typically falls between 400°C and 600°C (approximately 750°F to 1100°F). This specific range is not arbitrary; it is carefully controlled to balance the rate of thermal decomposition against the efficient removal of valuable liquid and gas products, a process fundamentally altered by the low-pressure environment.
The core challenge in pyrolysis is not just heating a material, but controlling the chemical reactions that follow. Vacuum pyrolysis leverages moderate temperatures combined with low pressure to selectively extract high-quality liquid fuels (bio-oil) by preventing them from degrading into less valuable char and gas.

The Principle: Heat, Decompose, Extract
What Happens During Pyrolysis?
Pyrolysis is the thermal decomposition of organic material in the absence of oxygen. When heated, long-chain polymer molecules in feedstocks like biomass, plastics, or tires become unstable and break apart.
The process creates three primary products: a solid residue (char), a condensable liquid (bio-oil or pyrolysis oil), and non-condensable gases (syngas).
Temperature's Direct Effect on Products
The final distribution of these three products is highly dependent on temperature and heating rate.
As a general rule, lower temperatures and slower heating rates favor the production of solid char. Conversely, very high temperatures favor the production of syngas. Fast and flash pyrolysis aim for a middle ground to maximize the liquid bio-oil.
How Vacuum Changes the Equation
The introduction of a vacuum fundamentally alters the process dynamics, allowing for greater control over the final products compared to pyrolysis at atmospheric pressure.
Lowering the Boiling Point
The primary function of the vacuum is to lower the pressure inside the reactor. This reduces the boiling point of the volatile compounds created as the feedstock decomposes.
Think of it like boiling water: at sea level, it boils at 100°C, but on a high mountain, where air pressure is lower, it boils at a much lower temperature. Vacuum pyrolysis applies this same principle to chemical vapors.
Suppressing Secondary Reactions
In conventional pyrolysis, the hot vapors linger in the reactor, where they can break down further (secondary cracking) into permanent gases or re-polymerize onto the solid surfaces to form more char.
The vacuum acts as a rapid extraction mechanism. It quickly pulls the primary vapors out of the hot reaction zone as soon as they form, giving them no time to undergo these undesirable secondary reactions.
The Impact on Bio-Oil Quality
This immediate removal of vapors is the key to the high-quality output of vacuum pyrolysis. The resulting bio-oil has a lower viscosity, lower oxygen content, and greater stability because its molecules have not been cracked and degraded by prolonged heat exposure.
Understanding the Trade-offs
Choosing the right temperature is a balancing act between reaction speed, product yield, and operational cost. There is no single "best" temperature; it is always relative to the desired outcome.
Temperature vs. Product Yield
The 400°C to 600°C range represents a critical window for product optimization.
- Below 400°C: Decomposition is too slow for most industrial applications, yielding primarily char.
- 450°C to 550°C: This is often the "sweet spot" for maximizing bio-oil yield. The temperature is high enough for rapid decomposition, and the vacuum is efficient at preserving the valuable liquid vapors.
- Above 600°C: The thermal energy becomes so high that it begins to crack the oil vapors into syngas, even with the vacuum's rapid extraction. This shifts the output away from liquids and towards gas.
The Cost of Vacuum
Implementing and maintaining a vacuum system adds significant complexity and cost to a pyrolysis reactor. It requires robust seals, powerful vacuum pumps, and higher energy consumption.
This investment is weighed against the increased yield and higher quality (and thus higher monetary value) of the bio-oil produced.
Feedstock Sensitivity
The ideal temperature can also shift based on the specific feedstock being processed. Different materials, such as wood, agricultural waste, or plastics, have different chemical compositions and decomposition kinetics, requiring fine-tuning of the process parameters.
Selecting the Right Temperature for Your Goal
Your target temperature within the vacuum pyrolysis range should be dictated by your primary objective. Use this as a guide for process design and optimization.
- If your primary focus is maximizing bio-oil yield: Operate in the middle of the range, typically 450°C to 550°C, to achieve a high rate of decomposition while minimizing secondary cracking of the vapors.
- If your primary focus is producing high-quality bio-char: Use the lower end of the range, around 400°C to 450°C. This maximizes solid yield while the vacuum allows for the recovery of valuable liquid co-products that would otherwise be lost.
- If your primary focus is co-producing syngas and liquids: Explore the upper end, from 550°C to 600°C or slightly higher. This intentionally promotes some thermal cracking to increase the gas fraction alongside a still-significant liquid yield.
Ultimately, temperature in vacuum pyrolysis is not a fixed number but a precise control dial for engineering your desired chemical output.
Summary Table:
| Temperature Range | Primary Goal | Key Outcome |
|---|---|---|
| 400°C - 450°C | Maximize Bio-Char Yield | High-quality char with liquid co-products |
| 450°C - 550°C | Maximize Bio-Oil Yield | Optimal balance of decomposition and vapor preservation |
| 550°C - 600°C+ | Co-produce Syngas & Liquids | Increased gas fraction alongside liquid yield |
Ready to optimize your pyrolysis process for superior bio-oil, char, or syngas yields? At KINTEK, we specialize in advanced laboratory equipment, including vacuum pyrolysis systems designed for precise temperature control and efficient product recovery. Whether you're processing biomass, plastics, or other feedstocks, our solutions help you achieve higher yields and better product quality. Contact our experts today to discuss how our lab equipment can enhance your research and development efforts!
Visual Guide
Related Products
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What are the different types of heat treatment process for steel? Tailor Strength, Hardness & Toughness
- What is a vacuum heat treatment furnace? The Ultimate Guide to Controlled Atmosphere Processing
- What are the four types of heat treating processes? Master Annealing, Normalizing, Hardening, and Tempering
- What are the parts of a vacuum furnace? A Guide to the 5 Core Systems
- What is the process of vacuum quenching? Achieve Superior Hardness with a Pristine Surface Finish



















