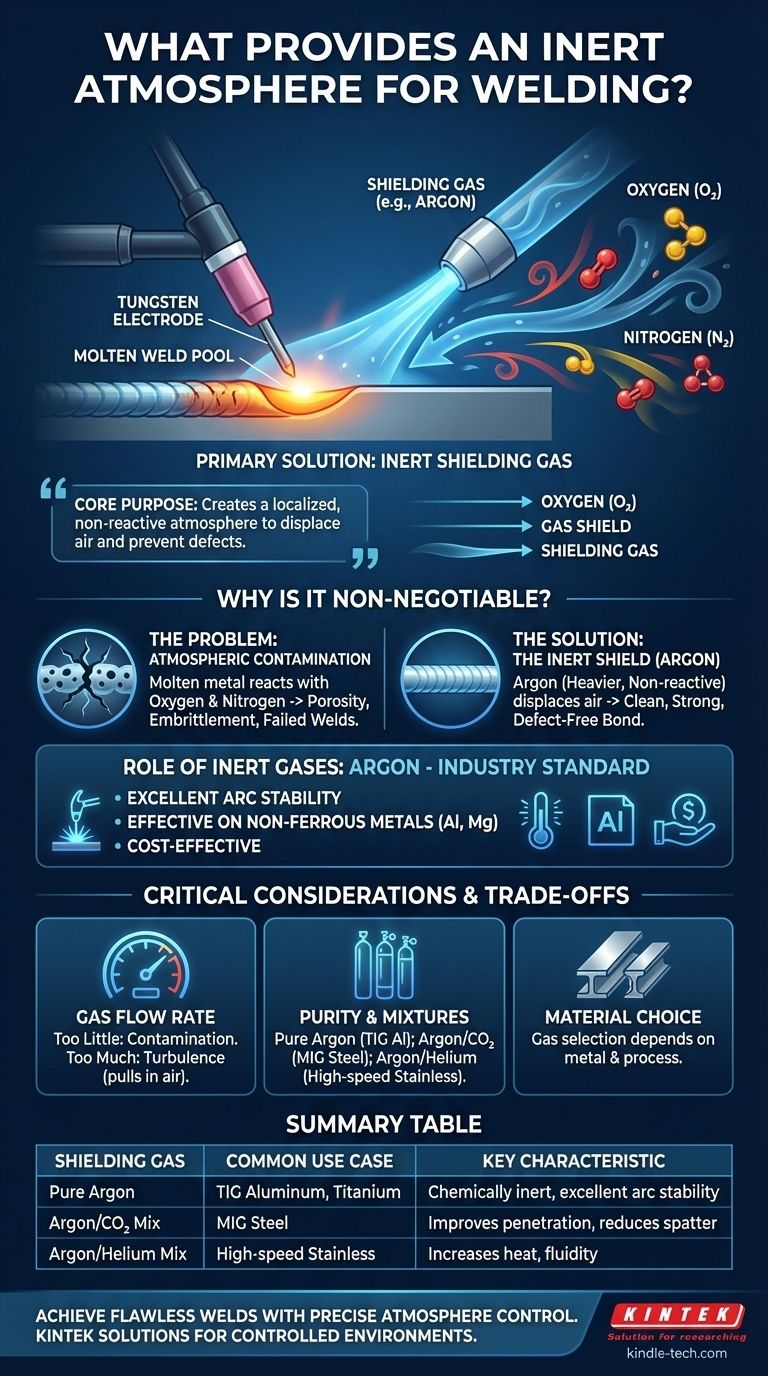
In welding, an inert atmosphere is created using a shielding gas, most commonly Argon. This noble gas is delivered from a pressurized cylinder through a hose to the welding torch. It flows out around the electrode and the weld area, physically displacing the surrounding air to prevent chemical reactions that would otherwise compromise the weld's integrity.
The core purpose of an inert shielding gas is not to add to the weld, but to fundamentally protect it. It creates a localized, non-reactive atmosphere that displaces oxygen and nitrogen, preventing catastrophic defects from forming in the molten weld pool.

Why a Shielding Gas is Non-Negotiable
To understand the role of Argon, you must first understand the primary enemy of a good weld: the atmosphere.
The Problem: Atmospheric Contamination
The air we breathe is approximately 78% nitrogen and 21% oxygen, with traces of water vapor and other gases. At room temperature, these gases are harmless to most metals.
However, at the extreme temperatures of a welding arc, molten metal is highly volatile and eager to react with these atmospheric elements.
The Consequences of Contamination
When molten metal is exposed to air, the oxygen and nitrogen react with the metal almost instantly. This contamination results in severe and unacceptable weld defects.
These defects include porosity (gas bubbles trapped within the weld) and embrittlement, both of which drastically reduce the strength and structural integrity of the final joint. A contaminated weld is a failed weld.
The Solution: An Inert Shield
A shielding gas like Argon solves this problem by creating a protective bubble around the arc and the molten weld pool.
Because Argon is heavier than air and chemically non-reactive (inert), it effectively pushes the oxygen and nitrogen away from the critical weld zone. This allows the molten metal to solidify into a clean, strong, and defect-free bond.
The Role of Inert Gases
The choice of shielding gas is critical and depends on the metal being welded and the desired outcome. The term "inert" is a key distinction.
The Definition of an Inert Gas
In chemistry, an inert gas is one that does not undergo chemical reactions under a given set of conditions. The noble gases, such as Argon and Helium, are the most common examples used in welding.
They are chosen precisely because they will not react with the molten metal, electrodes, or other materials in the high-temperature welding environment.
Argon: The Industry Standard
Argon is the most widely used inert shielding gas for several reasons. It provides excellent arc stability, is effective on a wide range of materials (especially non-ferrous metals like aluminum and magnesium), and is more cost-effective than other noble gases like Helium.
Understanding the Trade-offs
While the concept is simple, its practical application involves critical considerations that can make or break a project.
Gas Flow Rate
One of the most common mistakes is setting an incorrect flow rate. Too little flow will not provide adequate coverage, allowing atmospheric contamination to occur.
Conversely, too much flow is not only wasteful and expensive but can create turbulence. This turbulence can actually pull surrounding air into the weld zone, defeating the purpose of the shield entirely.
Purity and Mixtures
While pure Argon is essential for processes like TIG welding aluminum, other processes benefit from specific gas mixtures.
For example, small amounts of carbon dioxide are often mixed with Argon for MIG welding steel. This "active" gas improves arc stability and penetration, but the mixture is no longer purely inert. The choice always depends on the specific material and welding process.
Making the Right Choice for Your Goal
Selecting the correct shielding gas is fundamental to achieving a successful weld. Your decision should be guided by the material you are working with.
- If your primary focus is TIG welding non-ferrous metals (aluminum, magnesium, titanium): Pure Argon is the standard and correct choice to prevent contamination and ensure a clean weld.
- If your primary focus is MIG welding steel for better bead shape and less spatter: An Argon and Carbon Dioxide mixture (typically 75% Argon / 25% CO2) is the industry workhorse.
- If your primary focus is high-speed or deep-penetration welding on stainless steel: An Argon and Helium mixture may be required to increase the heat input and fluidity of the weld pool.
Ultimately, mastering the science of the shield is the first step toward mastering the art of the weld.
Summary Table:
| Shielding Gas | Common Use Case | Key Characteristic |
|---|---|---|
| Pure Argon | TIG Welding Aluminum, Titanium | Chemically inert, excellent arc stability |
| Argon/CO2 Mix | MIG Welding Steel | Improves penetration, reduces spatter |
| Argon/Helium Mix | High-speed or Deep Penetration Welding | Increases heat input and weld pool fluidity |
Achieve Flawless Welds with the Right Atmosphere Control
Understanding shielding gas is critical, but having the right equipment is what brings that knowledge to life. Whether your lab work involves advanced material joining or requires precise atmospheric control for other processes, KINTEK has the expertise and equipment to support your success.
We specialize in providing high-quality lab equipment and consumables, including reliable gas delivery systems essential for controlled environments. Let us help you eliminate contamination and ensure the integrity of your work.
Contact KINTEK today to discuss your specific laboratory needs and discover how our solutions can enhance your welding and material processing projects.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
People Also Ask
- How does a laboratory vacuum or atmosphere furnace ensure reliability during the pre-oxidation of alloy specimens?
- What is the function of sintering atmosphere? Control Chemistry for Superior Metal Parts
- How does an industrial high-temperature cyclic oxidation furnace simulate extreme conditions for aluminide coatings?
- What is the primary purpose of introducing a high-purity Argon (Ar) protective atmosphere during boriding? Expert Guide
- What is an oxidizing atmosphere? Key insights into oxidation reactions and applications
- How does a high-temperature atmosphere furnace influence Si-O-C ceramics? Control Your Pyrolysis Environment
- How does a high-temperature atmosphere furnace contribute to AlN/Si3N4 substrates? Unlock Superior Thermal Conductivity
- Why are high-temperature atmosphere furnaces necessary for confined metal zeolite catalysts? Precision Lab Solutions



















