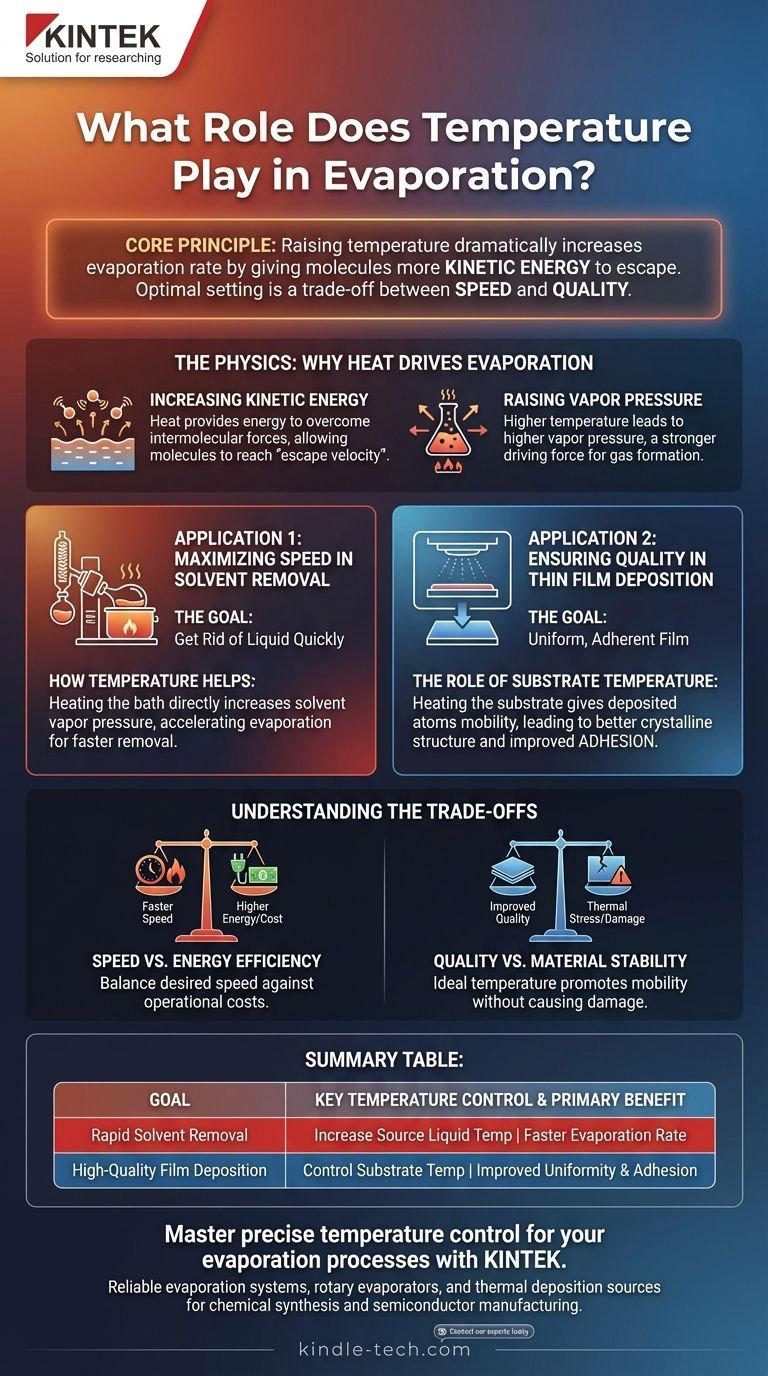
In short, raising the temperature dramatically increases the rate of evaporation. By giving molecules more kinetic energy, heat allows them to escape their liquid or solid state more easily. This core principle is fundamental, but its practical application depends entirely on whether your goal is to remove a solvent quickly or to deposit a high-quality material onto a surface.
Temperature is the primary control lever in any evaporation process. However, its optimal setting is a trade-off, forcing a choice between the speed of removal and the structural quality of a deposit.

The Physics: Why Heat Drives Evaporation
Increasing Kinetic Energy
At a molecular level, temperature is a measure of kinetic energy. For a molecule to evaporate, it must have enough energy to overcome the intermolecular forces holding it in a liquid or solid.
Increasing the temperature of a material means more of its individual molecules will reach this "escape velocity," causing the overall rate of evaporation to rise significantly.
Raising the Vapor Pressure
Vapor pressure is the pressure exerted by a vapor in equilibrium with its condensed phase (solid or liquid). When you heat a solvent, its vapor pressure increases.
A higher vapor pressure means the system is pushing harder to become a gas. This increased driving force results in faster net evaporation, which is the principle behind using a heated bath with a rotary evaporator.
Application 1: Maximizing Speed in Solvent Removal
The Goal: Get Rid of the Liquid
In many chemical processes, such as rotary evaporation or distillation, the primary objective is to remove a solvent from a solute as quickly as possible.
How Temperature Helps
Heating the liquid bath directly increases the solvent's vapor pressure, accelerating evaporation. A higher temperature will always remove the solvent faster.
Application 2: Ensuring Quality in Thin Film Deposition
The Goal: A Uniform, Adherent Film
In materials science and semiconductor manufacturing, evaporation is used to deposit a thin film of material onto a substrate. Here, the goal is not speed, but the quality, uniformity, and adhesion of the final film.
The Role of Substrate Temperature
In this context, the temperature of the substrate (the surface being coated) is just as important as the source material's temperature.
Properly heating the substrate gives the freshly deposited atoms enough energy to move around on the surface. This mobility allows them to settle into a more stable, uniform, and well-ordered crystalline structure.
Improving Adhesion
A heated substrate also promotes better adhesion between the deposited film and the surface. Heating the substrate above 150 °C, for example, can be critical for preventing the film from peeling or flaking off later.
Understanding the Trade-offs
Speed vs. Energy Efficiency
While a hotter bath evaporates solvent faster, it also consumes more energy and takes longer to heat up. For large-scale industrial processes, you must balance the desired speed of evaporation against the operational cost in time and electricity.
Quality vs. Material Stability
In film deposition, a higher substrate temperature improves film quality, but there is a limit. Excessive heat can introduce thermal stress into the film, cause unwanted chemical reactions, or even damage a sensitive substrate. The ideal temperature promotes mobility without causing damage.
How to Apply This to Your Goal
Choosing the correct temperature setting requires understanding your primary objective.
- If your primary focus is rapid solvent removal: Increase the source liquid's temperature as high as is safely possible, balancing the need for speed against energy consumption.
- If your primary focus is high-quality film deposition: Carefully control the substrate's temperature to provide just enough surface mobility for good film structure and adhesion, without damaging the substrate or the film itself.
Ultimately, mastering temperature is the key to controlling the outcome of any evaporation process.
Summary Table:
| Goal | Key Temperature Control | Primary Benefit |
|---|---|---|
| Rapid Solvent Removal | Increase Source Liquid Temperature | Faster evaporation rate |
| High-Quality Film Deposition | Control Substrate Temperature | Improved film uniformity and adhesion |
Master precise temperature control for your evaporation processes with KINTEK.
Whether you are focused on rapid solvent removal in a chemical synthesis or require the highest quality thin film deposition for semiconductor manufacturing, the right lab equipment is critical. KINTEK specializes in providing reliable evaporation systems, including rotary evaporators and thermal deposition sources, designed to deliver the precise temperature control your application demands.
Contact our experts today to discuss how our solutions can enhance your lab's efficiency, improve your results, and ensure process reproducibility.
Visual Guide
Related Products
- Automatic Laboratory Heat Press Machine
- Electric Heated Hydraulic Vacuum Heat Press for Lab
- Heated Hydraulic Press Machine with Heated Plates for Vacuum Box Laboratory Hot Press
- Vacuum Hot Press Furnace Machine Heated Vacuum Press
- Double Plate Heating Press Mold for Lab
People Also Ask
- What are the applications of electron beams? From Nanoscale Imaging to Industrial Manufacturing
- What is the use of physical vapor deposition? Enhance Durability, Performance, and Purity
- What are the drawbacks of thermal evaporation? Understanding the Limitations for High-Performance Applications
- What is electron beam physical vapor deposition method? A High-Purity Coating Technique for Demanding Applications
- What metal can evaporate? A Guide to Vapor Pressure and Thin Film Deposition
- What is the thermal process of evaporation? A Guide to High-Purity Thin Film Deposition
- Which parameter effect on thin film formation in thermal evaporation? Master the Key Variables for Superior Films
- How source material is evaporated during deposition? A Guide to Resistive vs. E-Beam Methods



















