For burning samples at high temperatures, the most common choices are porcelain and alumina (aluminum oxide) crucibles. Porcelain is a cost-effective workhorse for temperatures up to approximately 1150°C (2100°F), while alumina is preferred for its superior chemical inertness and higher temperature stability, often up to 1700°C (3090°F). The definitive choice, however, depends entirely on your specific temperature requirements and the chemical nature of your sample.
The term "high temperature" is relative. Selecting the correct crucible is not about finding a single "best" material, but about matching the crucible's properties—its maximum temperature, chemical inertness, and thermal shock resistance—to the precise demands of your analysis.

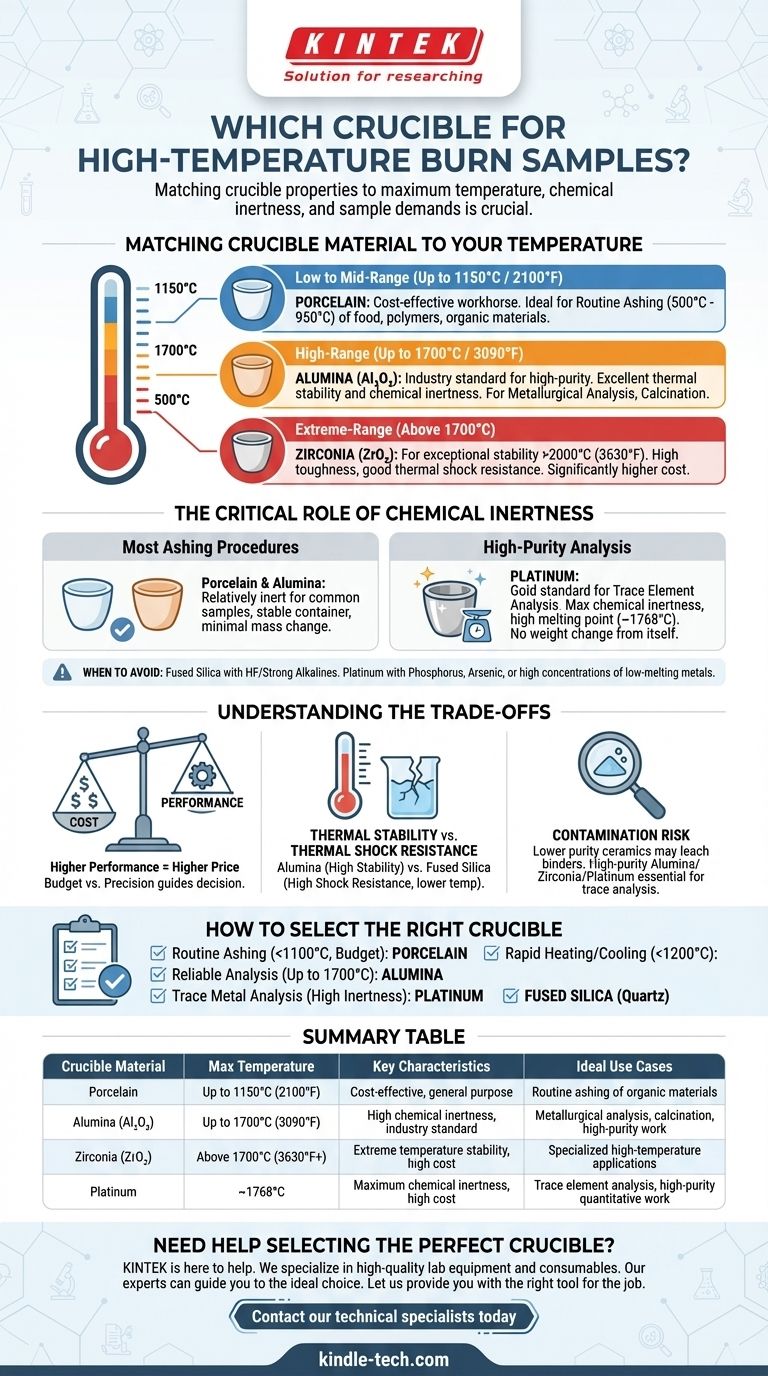
Matching the Crucible Material to Your Temperature
The first and most important filter for selecting a crucible is the maximum temperature of your procedure. Using a crucible above its recommended service temperature can lead to melting, cracking, or chemical reactions that will ruin your sample and your data.
Low to Mid-Range (Up to 1150°C / 2100°F): Porcelain
Glazed porcelain crucibles are the most common tool for general laboratory ashing and ignition tasks. They are inexpensive and readily available.
They are ideal for procedures like determining the ash content of food, polymers, or other organic materials where temperatures are typically held between 500°C and 950°C.
High-Range (Up to 1700°C / 3090°F): Alumina (Al₂O₃)
For temperatures beyond the limits of porcelain, high-purity alumina is the standard. It offers excellent thermal stability and is highly resistant to chemical attack.
This makes it the material of choice for metallurgical analysis, calcination of pigments, and working with glass melts. The higher the purity of the alumina (e.g., 99.7%+), the better its performance and inertness.
Extreme-Range (Above 1700°C): Zirconia (ZrO₂)
For applications requiring stability at even higher temperatures, zirconia crucibles are the solution. They can often be used in excess of 2000°C (3630°F).
Zirconia is exceptionally tough and offers good resistance to thermal shock, but it comes at a significantly higher cost than alumina.
The Critical Role of Chemical Inertness
A crucible must act as an inert container that does not react with, add to, or subtract from your sample. The success of a quantitative analysis, such as loss on ignition (LOI), depends on this principle.
For Most Ashing Procedures: Porcelain & Alumina
Both porcelain and alumina are relatively inert for most common sample types. They provide a stable container that will not significantly gain or lose mass during a typical burn-off procedure in air.
For High-Purity Analysis: Platinum
When performing trace element analysis or when absolute chemical inertness is required, platinum is the gold standard. It has a high melting point (~1768°C) and is exceptionally non-reactive with most chemicals.
Because platinum itself does not oxidize or change weight during heating, it ensures that any measured weight change comes only from your sample.
When to Avoid Certain Materials
No crucible is universally inert. For example, fused silica (quartz) should not be used with hydrofluoric acid or strong alkaline substances. Platinum can be attacked by samples containing phosphorus, arsenic, or high concentrations of lead or other low-melting-point metals at high temperatures.
Understanding the Trade-offs
Choosing a crucible involves balancing performance with practical constraints. An incorrect choice can be costly or, worse, invalidate your results.
Cost vs. Performance
There is a direct correlation between performance and price. A porcelain crucible may cost only a few dollars, while a similarly sized platinum crucible can cost thousands. Your budget and the required analytical precision will guide your decision.
Thermal Stability vs. Thermal Shock Resistance
Materials with the highest temperature stability, like alumina, can be sensitive to thermal shock—cracking caused by rapid temperature changes. Materials like fused silica (quartz) offer outstanding thermal shock resistance but have a lower maximum operating temperature (around 1200°C).
Contamination Risk
Lower-purity ceramic crucibles can contain binders or silicates that may leach into a sample at high temperatures, which is a major concern for trace analysis. For these applications, investing in a high-purity alumina, zirconia, or platinum crucible is essential.
How to Select the Right Crucible
Base your decision on the specific goals of your work. Answering these questions first will lead you to the correct choice.
- If your primary focus is routine ashing below 1100°C on a budget: Porcelain is your most cost-effective and practical choice.
- If you need reliable, repeatable results for analysis at temperatures up to 1700°C: High-purity alumina is the industry standard.
- If your work involves trace metal analysis and requires the highest possible inertness: Platinum is the necessary investment, provided your sample does not contain elements that attack it.
- If your process involves extremely rapid heating and cooling cycles: Fused silica (quartz) is superior, as long as your temperature remains below 1200°C.
By first defining your maximum temperature, chemical environment, and analytical goal, you can select a crucible with confidence.
Summary Table:
| Crucible Material | Max Temperature | Key Characteristics | Ideal Use Cases |
|---|---|---|---|
| Porcelain | Up to 1150°C (2100°F) | Cost-effective, general purpose | Routine ashing of organic materials (food, polymers) |
| Alumina (Al₂O₃) | Up to 1700°C (3090°F) | High chemical inertness, industry standard | Metallurgical analysis, calcination, high-purity work |
| Zirconia (ZrO₂) | Above 1700°C (3630°F+) | Extreme temperature stability, high cost | Specialized high-temperature applications |
| Platinum | ~1768°C | Maximum chemical inertness, high cost | Trace element analysis, high-purity quantitative work |
Need Help Selecting the Perfect Crucible?
Choosing the right crucible is critical for accurate, repeatable results. The wrong material can lead to sample contamination, crucible failure, and ruined experiments.
KINTEK is here to help. We specialize in supplying high-quality lab equipment and consumables, including a full range of crucibles for every application and budget. Our experts can guide you to the ideal choice based on your specific temperature requirements, sample type, and analytical goals.
Let us provide you with the right tool for the job.
Contact our technical specialists today for a personalized consultation and ensure your high-temperature processes are a success.
Visual Guide
Related Products
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
People Also Ask
- What are the benefits of using an alumina crucible with a lid for TiB2 nanopowder heat treatment? Ensure High Purity
- How much heat can a ceramic crucible withstand? A Guide to Material-Specific Temperature Limits
- What is the temperature range of alumina crucibles? Key Factors for Safe High-Temp Use
- What precautions should be taken when using a crucible? Essential Steps for Safety and Accuracy
- What is a crucible material for a furnace? A Guide to Choosing the Right High-Temperature Container



















