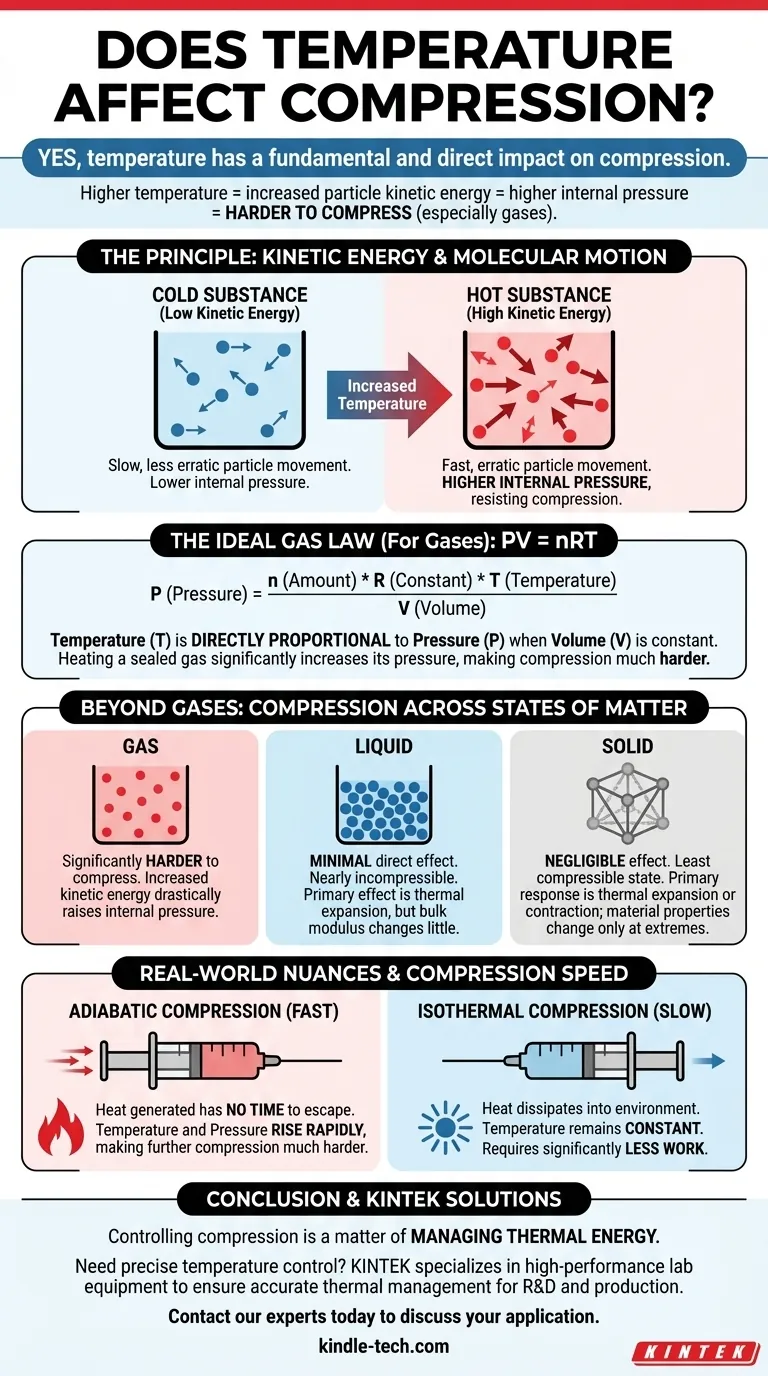
Yes, temperature has a fundamental and direct impact on compression. For gases in particular, as you increase the temperature of a substance, its particles gain kinetic energy, move faster, and push outwards with greater force. This increased internal pressure makes the substance significantly harder to compress.
The core principle is a direct relationship between energy and pressure. Higher temperature means more particle energy, which creates higher internal pressure that you must overcome to compress a substance, especially a gas. This relationship is a cornerstone of thermodynamics.

The Principle: Kinetic Energy and Molecular Motion
To understand why temperature affects compression, we need to look at what's happening at the molecular level.
What Temperature Represents
Temperature is not an abstract property; it's a direct measure of the average kinetic energy of the atoms or molecules within a substance.
Hotter particles move faster and more erratically. Colder particles move more slowly.
How Kinetic Energy Resists Compression
When you compress a substance, you are forcing its particles closer together. The kinetic energy of these particles creates an internal pressure that pushes back against this external force.
In a hot gas, the fast-moving particles collide with the walls of their container more frequently and with greater force. To reduce the volume, you must apply a significantly larger external force to overcome this powerful internal pressure.
Quantifying the Relationship: The Ideal Gas Law
For gases, this relationship is elegantly described by a foundational principle in physics and chemistry.
The Formula: PV = nRT
The Ideal Gas Law provides a mathematical model for the behavior of most gases under common conditions. The formula is PV = nRT, where:
- P is the pressure
- V is the volume
- n is the amount of the gas
- R is the ideal gas constant
- T is the temperature
Temperature's Direct Role
In this equation, temperature (T) is directly proportional to pressure (P) if the volume is held constant.
This means if you take a sealed container of air and heat it, the pressure inside will rise. This increased pressure is the very resistance you feel when trying to compress a hot gas.
A Simple Analogy: A Bicycle Pump
When you use a hand pump to inflate a tire, you are rapidly compressing air. You'll notice the pump barrel gets hot.
This isn't just from friction. You are doing work on the gas, which increases its internal energy and therefore its temperature. This effect, known as adiabatic heating, makes the air harder to compress as you pump faster.
Beyond Gases: Liquids and Solids
While the effect is most dramatic in gases, temperature also influences the compression of liquids and solids, though in different ways.
The Case of Liquids
Liquids are considered nearly incompressible. Their molecules are already in close contact, leaving little free space.
Temperature's main effect on a liquid is thermal expansion. Heating a liquid will cause it to expand slightly, but its resistance to compression (its bulk modulus) does not change as dramatically as it does for a gas.
The Behavior of Solids
Solids are the least compressible state of matter. Like liquids, their primary response to temperature change is thermal expansion or contraction.
While extreme temperatures can affect a solid's material properties like stiffness, the direct impact on its compressibility is negligible in most engineering scenarios compared to the effect on gases.
Common Pitfalls and Real-World Nuances
The Ideal Gas Law is a powerful model, but real-world applications have important complexities.
Ideal Gas vs. Real Gas
The Ideal Gas Law assumes gas particles have no volume and no intermolecular attractions. This is a useful simplification, but real gases deviate from this model at very high pressures or very low temperatures.
Adiabatic vs. Isothermal Compression
The speed of compression matters immensely.
- Adiabatic Compression (Fast): When you compress a gas quickly, the heat generated has no time to escape. This temperature increase raises the internal pressure, making further compression much harder.
- Isothermal Compression (Slow): If you compress a gas very slowly, heat can dissipate into the environment, keeping the temperature constant. This requires significantly less work than adiabatic compression.
Making the Right Choice for Your Goal
Your approach depends entirely on what you are trying to achieve.
- If your primary focus is engineering a pneumatic or hydraulic system: You must actively manage heat. The temperature increase from rapid compression (adiabatic heating) will significantly increase the required force and can affect seals and fluid viscosity.
- If your primary focus is managing sealed, pressurized containers: You must account for ambient temperature swings. A tank filled on a cool morning will experience a significant pressure increase in the afternoon sun, which can become a critical safety factor.
- If your primary focus is understanding the core physics: Start with the Ideal Gas Law (PV=nRT). It is the essential model for grasping the direct and predictable relationship between temperature, pressure, and volume.
Ultimately, temperature is a form of energy, and controlling compression is a matter of managing that energy.
Summary Table:
| State of Matter | Effect of Increased Temperature on Compression | Key Principle |
|---|---|---|
| Gas | Significantly harder to compress | Ideal Gas Law (PV=nRT); increased kinetic energy raises internal pressure. |
| Liquid | Minimal direct effect on compressibility (nearly incompressible) | Primary effect is thermal expansion; bulk modulus changes little. |
| Solid | Negligible effect on compressibility in most scenarios | Primary effect is thermal expansion/contraction; material properties may change at extremes. |
Need precise temperature control for your compression processes?
Understanding the thermodynamics of compression is crucial for R&D, quality control, and process optimization. KINTEK specializes in high-performance lab equipment, including ovens, furnaces, and temperature control systems, designed to help you accurately manage thermal energy in your experiments and production.
Let our experts help you select the right equipment to ensure reliable and repeatable results. Contact our technical team today to discuss your specific application needs.
Visual Guide
Related Products
- Electric Lab Cold Isostatic Press CIP Machine for Cold Isostatic Pressing
- Automatic Lab Cold Isostatic Press CIP Machine Cold Isostatic Pressing
- Manual Cold Isostatic Pressing Machine CIP Pellet Press
- Warm Isostatic Press WIP Workstation 300Mpa for High Pressure Applications
- Manual High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
People Also Ask
- What is the primary function of an ultrasonic generator in graphite exfoliation? Unlock High-Quality Graphene Production
- How does the refrigeration system of an Ultra Freezer work? The Two-Stage Cascade Cooling Explained
- What industrial uses are there for diamonds? Unlock High-Performance Applications
- Does strain hardening affect conductivity? Understanding the Strength vs. Conductivity Trade-Off
- What is the function of a laboratory vacuum system in preparing COF precursors? Ensure Purity & Prevent Oxidation
- Do diamond testers really work? Uncover the truth about their accuracy and limitations.
- How does a high-power ultrasonic homogenizer assist in the preparation of organic-inorganic nanocomposites?
- What is the cheapest type of additive manufacturing process? Start 3D Printing on a Budget with FDM



















