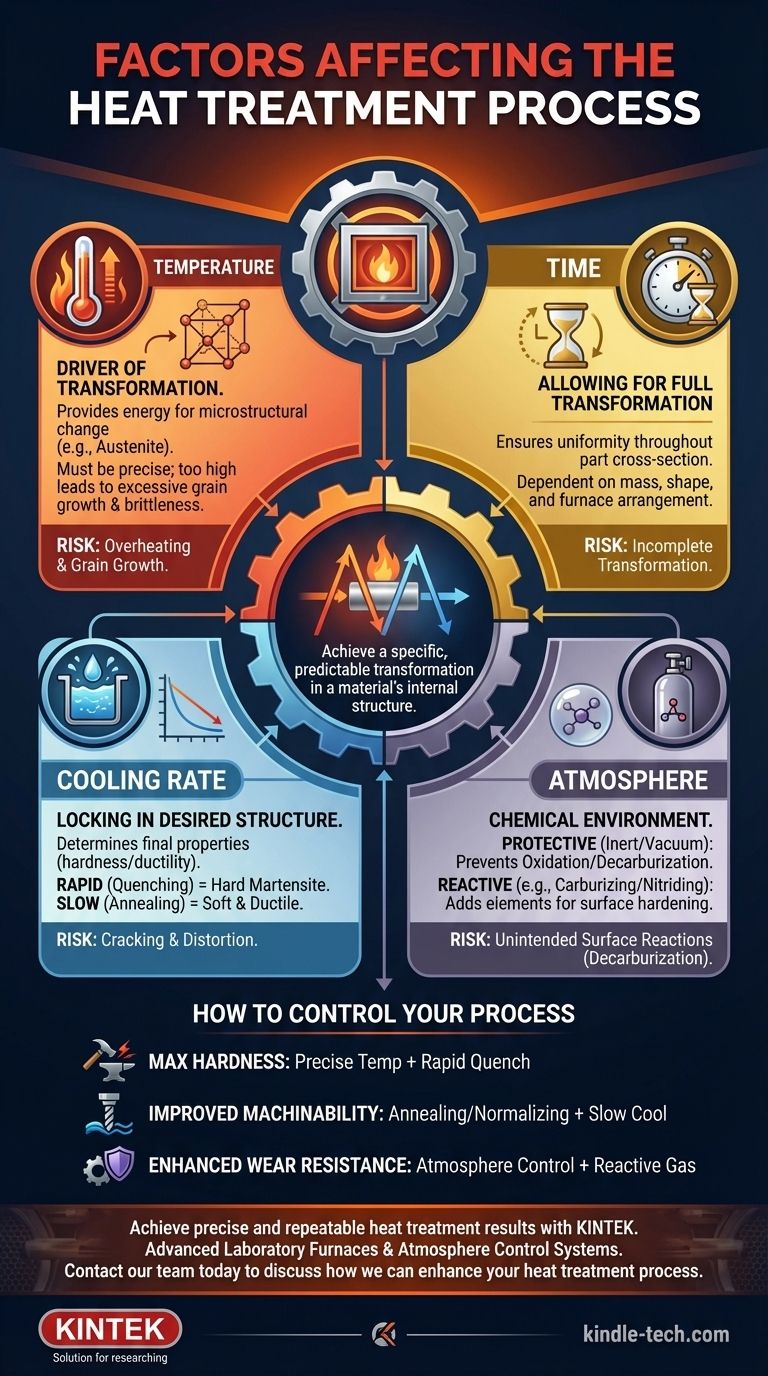
The success of any heat treatment process hinges on the precise control of four fundamental factors. These are the heating temperature, the holding time at that temperature, the cooling rate, and the composition of the furnace atmosphere. Each variable directly influences the final microstructure of the material, which in turn dictates its mechanical properties like hardness, toughness, and ductility.
Heat treatment is not simply a heating and cooling cycle. It is a controlled metallurgical process where the interplay between temperature, time, cooling, and atmosphere is manipulated to achieve a specific, predictable transformation in a material's internal structure.

The Core Pillars of Heat Treatment
To achieve consistent and reliable results, you must understand how each primary factor drives metallurgical change within the workpiece. These are not independent variables; they are deeply interconnected.
Temperature: The Driver of Transformation
Temperature provides the thermal energy necessary to initiate changes in the material's crystal structure, or microstructure. For steels, this typically involves heating to a temperature where the structure transforms into austenite.
The chosen temperature must be high enough to cause the desired transformation but controlled to prevent negative effects like excessive grain growth, which can make the material brittle.
Time: Allowing for Full Transformation
Holding time is the duration the workpiece is kept at the peak temperature. Its purpose is to ensure that the thermal and chemical changes occur uniformly throughout the entire cross-section of the part.
This is not a fixed number. It is directly influenced by the workpiece's mass, shape, and arrangement in the furnace. Larger loads or complex parts with thick sections require longer holding times to ensure the core reaches the same temperature as the surface.
Cooling Rate: Locking in the Desired Structure
The rate at which the material is cooled from the treatment temperature determines the final microstructure and, therefore, its properties. This is arguably the most critical step in defining the outcome.
A rapid cool, or quenching (e.g., in water or oil), traps the material in a hard, brittle state like martensite. A slow cool, or annealing (e.g., letting it cool in the furnace), allows the structure to rearrange into a soft, ductile state.
Atmosphere: The Chemical Environment
The gas inside the furnace is not just a medium for heat transfer; it is an active chemical agent. The furnace atmosphere serves one of two primary functions.
First, it can be protective. A vacuum or an inert gas like argon prevents surface reactions like oxidation (scaling) and decarburization, preserving the workpiece's surface integrity.
Second, it can be reactive. Gaseous mediums can be intentionally introduced to chemically alter the surface of the part, as seen in processes like carburizing or nitriding, which add carbon or nitrogen to create a hard, wear-resistant case.
Understanding the Trade-offs and Risks
A failure to control any of the core factors can lead to failed parts, wasted resources, and inconsistent performance. Understanding these common failure modes is essential for process control.
Overheating and Grain Growth
Using a temperature that is too high or a holding time that is too long can cause the crystalline grains within the metal to grow excessively large. This permanently reduces the material's toughness and ductility, making it brittle even if the hardness reading seems correct.
Incomplete Transformation
Insufficient holding time or temperature means the core of the part never fully transforms. This results in a component with a hard surface but a soft, weak core, leading to premature failure under load.
Cracking and Distortion
The most common cause of cracking is a cooling rate that is too severe for the part's geometry. The extreme thermal stress created when the surface contracts much faster than the core can physically tear the material apart.
Unintended Surface Reactions
If the furnace atmosphere is not properly controlled, unwanted chemical reactions will occur. Decarburization, the loss of carbon from the surface of steel, makes the surface soft and unable to achieve the desired hardness, compromising wear resistance.
How to Control Your Process
Your approach to heat treatment must be dictated by your end goal for the material. Different properties require different combinations of these core factors.
- If your primary focus is maximizing hardness: You need precise temperature control to form full austenite, followed by a rapid and aggressive quench designed for that specific alloy.
- If your primary focus is improving machinability (softening): You will use an annealing or normalizing process, which involves heating the part and then ensuring a very slow and controlled cooling rate.
- If your primary focus is enhancing surface wear resistance: Your critical variable is the furnace atmosphere, using an active gas mixture for carburizing or nitriding to harden only the surface layer.
Mastering the deliberate control of these variables is the key to unlocking the full potential of your materials.
Summary Table:
| Factor | Role in the Process | Key Consideration |
|---|---|---|
| Temperature | Drives microstructural transformation (e.g., to austenite) | Must be high enough for transformation but controlled to prevent grain growth. |
| Holding Time | Ensures uniform transformation throughout the part | Depends on part mass, shape, and furnace load. |
| Cooling Rate | Determines final microstructure and properties (hardness/ductility) | Ranges from rapid quenching (martensite) to slow annealing. |
| Furnace Atmosphere | Protects the surface or chemically alters it (e.g., carburizing) | Can be inert (protective) or reactive (surface hardening). |
Achieve precise and repeatable heat treatment results with KINTEK.
Whether your goal is maximum hardness, improved machinability, or superior surface wear resistance, the right equipment is essential for controlling temperature, time, cooling rate, and atmosphere. KINTEK specializes in advanced laboratory furnaces and atmosphere control systems designed for metallurgical research and production.
Let our experts help you select the perfect solution for your specific material and application needs. Contact our team today to discuss how we can enhance your heat treatment process.
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- Vertical Laboratory Tube Furnace
People Also Ask
- What is the primary purpose of introducing a high-purity Argon (Ar) protective atmosphere during boriding? Expert Guide
- How to create an inert atmosphere in a furnace? Master the Vacuum-Purge Method for Oxidation-Free Results
- What is the primary role of a high-temperature atmosphere furnace in the production of activated carbon xerogels?
- What is the dew point of a sintering furnace? A Key to Preventing Oxidation & Ensuring Quality
- What is hydrogen annealing? Achieve Superior Material Properties with Bright Annealing
- What are the dangers of inert gases? The Silent, Undetectable Threat of Oxygen Displacement
- Why is an atmosphere sintering furnace required for LiNbO3-coated NMC811? Optimize High-Nickel Cathode Performance
- Can nitrogen gas be heated? Leverage Inert Heat for Precision and Safety



















