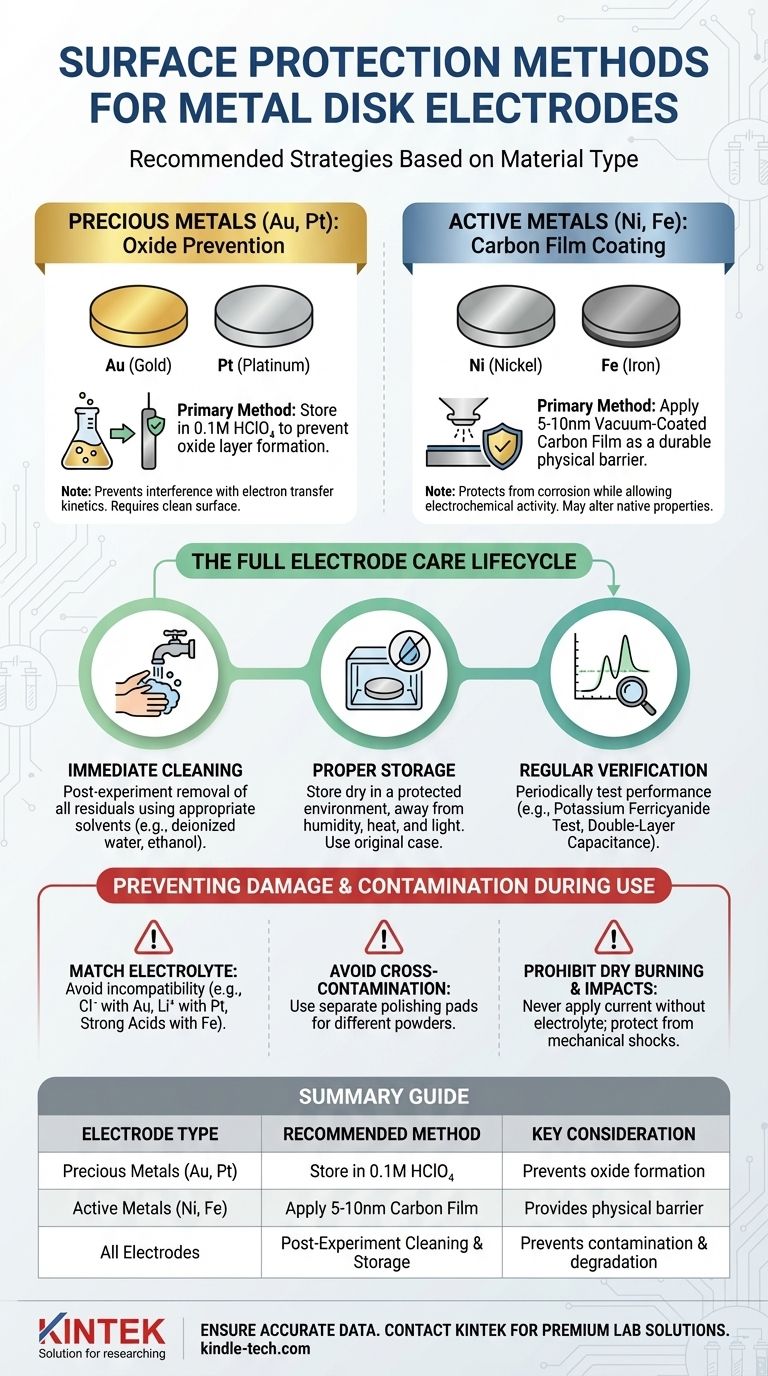
The most effective surface protection for a metal disk electrode depends entirely on its material. For precious metals like gold or platinum, prevention of surface oxide formation is key, which is best achieved by storing the electrode in 0.1M HClO₄. For more reactive, active metals such as nickel, a physical barrier like a 5-10nm vacuum-coated carbon film offers the most robust protection.
Protecting an electrode's surface is not a single action but a comprehensive lifecycle. True protection involves choosing the right method for the material, preventing damage during use, and following a strict cleaning and storage protocol to ensure long-term performance and data reliability.

Foundational Protection Strategies
The initial choice of protection is dictated by the chemical reactivity of the electrode material itself. The goal is always to preserve a clean, electrochemically active surface, but the approach differs significantly between inert and active metals.
For Precious Metals (Au, Pt): Oxide Prevention
Precious metals like gold and platinum are relatively inert but can still form a thin oxide layer on their surface when exposed to air.
This oxide film can interfere with electron transfer kinetics, leading to inaccurate and non-reproducible experimental results.
To prevent this, immersing the electrode in a 0.1M perchloric acid (HClO₄) solution during storage is the recommended practice.
For Active Metals (Ni, Fe): Carbon Film Coating
Active metals like nickel, iron, or copper are much more susceptible to oxidation and corrosion. Simple immersion is often insufficient for long-term protection.
Applying a very thin (5-10nm) carbon film via vacuum coating creates a durable physical barrier.
This film protects the underlying metal from aggressive environments while still allowing for electrochemical activity, though it may alter the surface's native properties.
Preventing Damage and Contamination During Use
Protection extends beyond storage. The most common causes of electrode failure are preventable mistakes made during the experimental process. Adhering to strict operational protocols is critical for electrode longevity.
Match the Electrolyte to the Electrode
Electrolyte compatibility is non-negotiable. Using an incompatible electrolyte is a guaranteed way to corrode or damage the electrode surface.
For example, avoid electrolytes containing chloride ions with gold electrodes and lithium ions with platinum electrodes. Similarly, strong acids should not be used with iron-based electrodes.
Avoid Cross-Contamination During Polishing
If you polish your electrodes to restore the surface, you must use different polishing pads for different polishing powders.
Reusing a pad introduces abrasive particles from a previous step, which can scratch the surface and contaminate the electrode, compromising your results.
Prohibit Dry Burning and Impacts
Never apply a current to the electrode without an electrolyte present (dry burning), as this can cause irreversible damage to the surface.
The electrode surface is also fragile. Protect it from mechanical impacts, drops, or collisions with other lab equipment.
Understanding the Trade-offs and Pitfalls
While protection methods are essential, they are not without their own considerations. Understanding their limitations is key to making informed decisions and interpreting your data correctly.
The Impact of Protective Coatings
A physical coating, like the carbon film used on active metals, inherently changes the electrode's surface.
While it provides excellent protection, this film can alter electron transfer rates and the electrode's electrochemical signature compared to the bare metal. This is a critical trade-off between preservation and maintaining a native surface.
The Limits of Chemical Immersion
Storing a precious metal electrode in HClO₄ prevents new oxide formation, but it does not repair a surface that is already oxidized or contaminated.
This method is part of a maintenance routine, not a solution for a damaged or dirty electrode. Proper cleaning after each experiment must precede storage.
Ignoring Gradual Degradation
Electrode failure is rarely sudden. It is a slow process of contamination, surface roughening, or passivation.
Relying on visual inspection alone is not enough. You must actively verify the electrode's performance to catch this gradual decline before it invalidates your research.
The Full Lifecycle of Electrode Care
A truly protected electrode is one that is managed properly from the end of one experiment to the beginning of the next. This requires a disciplined, three-step process.
Step 1: Immediate Post-Experiment Cleaning
As soon as an experiment is complete, remove the electrode from the apparatus.
Thoroughly clean the surface with appropriate solvents, such as deionized water or ethanol, to remove all residual electrolyte and reaction products.
Step 2: Proper Storage
After cleaning, ensure the electrode is completely dry.
Store it in a dry, protected environment away from humidity, high temperatures, and strong light. Using the original case it came in is always the best practice.
Step 3: Regular Performance Verification
Periodically test the electrode's performance to ensure it meets specifications. Two standard verification methods are:
- Potassium Ferricyanide Test: The peak potential separation (ΔEp) should be less than or equal to 80mV at a 100mV/s scan rate.
- Double-Layer Capacitance: The measurement fluctuation in a 0.1M KCl solution should be less than 15%.
Making the Right Choice for Your Goal
- If your primary focus is working with precious metals like Gold or Platinum: Prioritize preventing surface oxides through proper storage in 0.1M HClO₄ and meticulous electrolyte selection.
- If your primary focus is using active metals like Nickel or Iron: Consider a protective vacuum-coated carbon film for robust protection, but be aware of its potential impact on surface kinetics.
- If your primary focus is maximum data accuracy and reproducibility: Implement a rigorous protocol of post-experiment cleaning, proper storage, and regular performance verification to catch degradation early.
Ultimately, consistent and methodical care is the foundation of reliable and reproducible electrochemical data.
Summary Table:
| Electrode Type | Recommended Protection Method | Key Consideration |
|---|---|---|
| Precious Metals (Au, Pt) | Store in 0.1M HClO₄ | Prevents oxide formation |
| Active Metals (Ni, Fe) | Apply 5-10nm carbon film | Provides physical barrier |
| All Electrodes | Post-experiment cleaning & storage | Prevents contamination & degradation |
Ensure your electrochemical data is accurate and reproducible with KINTEK's premium lab equipment and consumables. We specialize in providing reliable solutions for all your laboratory needs, including electrode care products. Contact us today to learn how we can support your research with high-quality equipment and expert guidance!
Visual Guide
Related Products
- RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Warm Isostatic Press for Solid State Battery Research
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Graphite Vacuum Continuous Graphitization Furnace
People Also Ask
- Why Use a Three-Electrode RDE System for PEM Catalyst Screening? Master Intrinsic Kinetic Activity Analysis
- Why is a high-precision Rotating Ring-Disk Electrode (RRDE) essential for ORR? Unlock Precise Catalytic Kinetics
- What are the technical advantages of RRDE for electrochemical studies? Unlock Real-Time Intermediate Detection
- What role does the RRDE play in catalyst evaluation for H2O2 synthesis? Enhance Selectivity and Kinetic Precision
- What is the rotating ring disk electrode method? Unlock Real-Time Reaction Analysis















