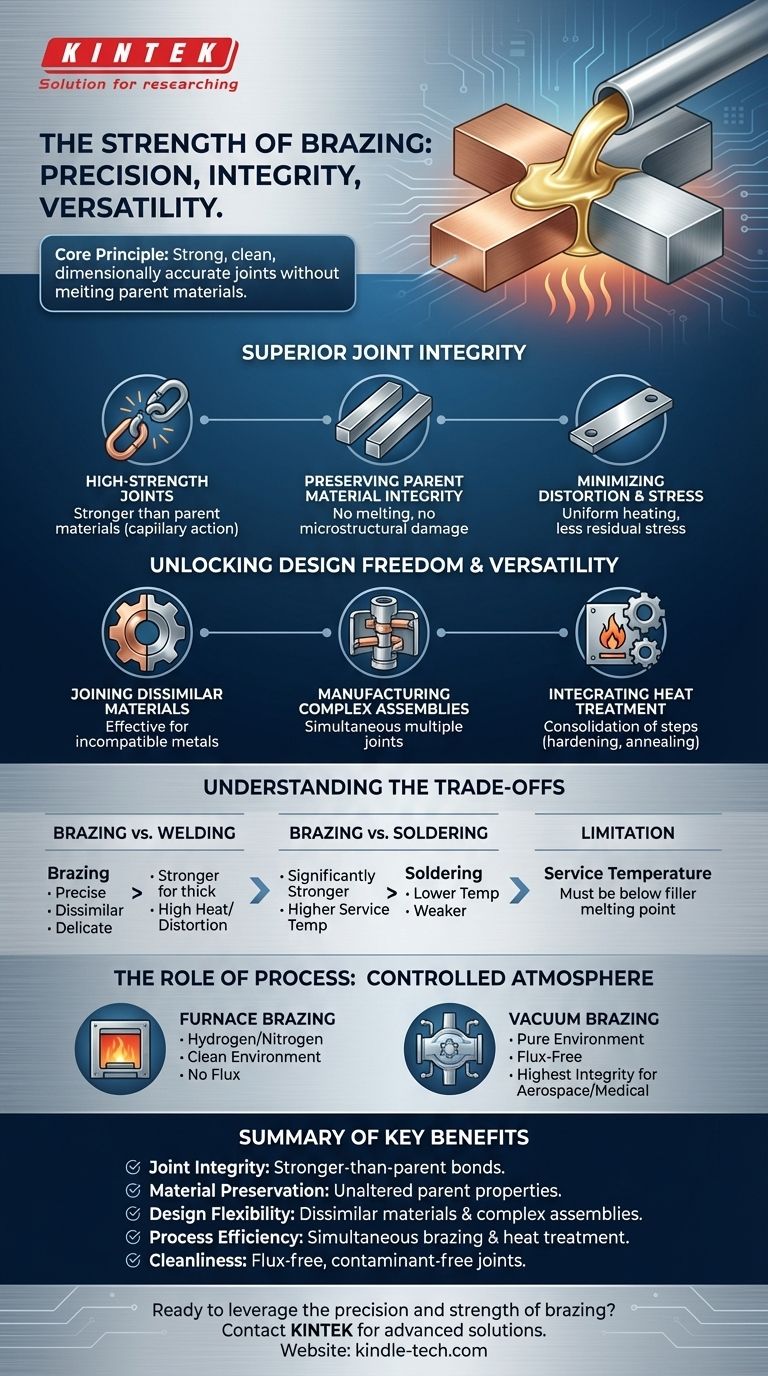
At its core, brazing is a joining process prized for its ability to create strong, clean, and dimensionally accurate assemblies. Unlike welding, which melts the base materials, brazing uses a filler metal that melts at a lower temperature. This filler is drawn into a tight-fitting joint by capillary action, creating a powerful metallurgical bond upon cooling without distorting or damaging the parent components.
The true strength of brazing is not merely joining metals, but doing so with precision and finesse. It excels where welding would be too destructive or imprecise, enabling the creation of complex, multi-material assemblies while preserving the integrity of the original components.

Why Choose Brazing? Superior Joint Integrity
The primary benefit of brazing lies in the quality of the joint it produces. Because the process operates below the melting point of the parent materials, it avoids the significant drawbacks associated with high-heat fusion methods.
Creating High-Strength Joints
Brazed joints are exceptionally strong. The filler metal forms a metallurgical bond with the parent materials, and when properly designed, the resulting joint can be stronger than the parent materials themselves.
The strength comes from the capillary action that pulls the molten filler metal into the entire gap between the components, ensuring a complete, void-free connection.
Preserving Parent Material Integrity
Since the parent metals are only heated and never melted, their fundamental mechanical and physical properties remain intact.
This is a critical advantage over welding, which can alter the microstructure of the heat-affected zone, potentially weakening the material or changing its characteristics.
Minimizing Distortion and Internal Stress
Modern brazing, especially furnace brazing, uses uniform heating and controlled cooling cycles. This even distribution of thermal energy across the entire assembly drastically reduces distortion and locks in less residual stress.
The result is a more dimensionally stable and reliable part, which is crucial for high-precision components.
Unlocking Design Freedom and Versatility
Brazing offers engineers and designers a level of flexibility that is difficult to achieve with other joining methods. It opens the door to creating more intricate and optimized products.
Joining Dissimilar Materials
Brazing is one of the most effective methods for joining dissimilar metals, such as copper to steel or aluminum to stainless steel.
Because the parent metals are not melted together, their metallurgical incompatibility is not an issue. The filler metal is selected to be compatible with both materials, acting as a robust bridge between them.
Manufacturing Complex Assemblies
The process is ideal for creating complex parts that would be difficult or impossible to machine from a single block.
Engineers can braze multiple joints simultaneously in a single furnace cycle, including those in long or inaccessible locations. This allows for the production of delicate and intricate geometries with high repeatability.
Integrating Heat Treatment
Furnace and vacuum brazing cycles can be designed to incorporate heat treatment processes.
Processes like hardening, annealing, or age hardening can be performed in the same thermal cycle as the brazing itself. This consolidation saves significant time, handling, and cost by eliminating separate manufacturing steps.
Understanding the Trade-offs
No process is perfect for every application. Understanding where brazing excels—and where it doesn't—is key to making an informed decision.
Brazing vs. Welding
Welding typically produces stronger joints on a pound-for-pound basis and is better for thick sections. However, it introduces immense heat, causing distortion and altering material properties. Brazing is the superior choice for precision, dissimilar materials, and delicate assemblies.
Brazing vs. Soldering
Soldering is a similar process but occurs at a lower temperature (below 450°C / 840°F). Brazed joints are significantly stronger and have a higher service temperature than soldered joints, making brazing suitable for more demanding structural applications.
When Brazing Might Not Be the Best Fit
The primary limitation of a brazed joint is its service temperature, which must remain well below the melting point of the filler alloy. Furthermore, for applications requiring the absolute maximum joint strength in thick, simple sections, a properly executed weld may be preferable.
The Role of Process: Furnace and Vacuum Brazing
The environment in which brazing occurs is critical to its success. Modern controlled-atmosphere methods have eliminated many of the old challenges.
The Advantage of a Controlled Atmosphere
Furnace brazing takes place in a clean atmosphere (like hydrogen or nitrogen) or a vacuum. This environment prevents the formation of oxides during heating, which is crucial for proper filler metal flow.
This eliminates the need for corrosive chemical fluxes and the subsequent post-braze cleaning, resulting in a clean, bright part straight out of the furnace.
Vacuum Brazing: The Gold Standard
Vacuum brazing represents the pinnacle of brazing cleanliness and quality. By removing virtually all gases, it creates an exceptionally pure environment.
This process produces flux-free joints of the highest integrity, making it the standard for mission-critical applications in the aerospace, medical, and semiconductor industries.
Making the Right Choice for Your Application
Selecting the right joining method depends entirely on your project's specific goals.
- If your primary focus is joining dissimilar or delicate materials: Brazing is ideal because it operates below the melting point of the parent metals, preventing damage and enabling multi-material designs.
- If your primary focus is high-volume production of complex parts: Automated furnace brazing is highly efficient, allowing for multiple joints to be made simultaneously with excellent repeatability and minimal labor.
- If your primary focus is maximum joint purity and strength for critical applications: Vacuum brazing provides a flux-free, contaminant-free environment essential for creating the highest-integrity joints.
Ultimately, choosing brazing is a strategic decision to prioritize material integrity, design flexibility, and process cleanliness.
Summary Table:
| Strength of Brazing | Key Benefit |
|---|---|
| Joint Integrity | Creates stronger-than-parent-material bonds without melting the base metals. |
| Material Preservation | Avoids altering parent material properties, unlike welding. |
| Design Flexibility | Enables joining of dissimilar metals and complex, multi-part assemblies. |
| Process Efficiency | Allows simultaneous joint brazing and heat treatment in a single cycle. |
| Cleanliness | Vacuum/furnace brazing eliminates flux, producing contaminant-free joints. |
Ready to leverage the precision and strength of brazing for your lab or production needs? KINTEK specializes in advanced brazing solutions, including furnace and vacuum brazing systems, to help you join dissimilar materials, create complex assemblies, and achieve high-integrity joints with minimal distortion. Our expertise ensures your projects benefit from clean, reliable, and efficient joining processes. Contact us today to discuss how KINTEK's lab equipment and consumables can enhance your manufacturing capabilities!
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Brazing Furnace
- Boron Nitride (BN) Ceramic Rod for High Temperature Applications
- Advanced Engineering Fine Ceramics Boron Nitride (BN) Ceramic Parts
- Conductive Boron Nitride BN Ceramics Composite for Advanced Applications
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
People Also Ask
- What are the advantages of brazing compared to welding? Achieve Clean, Low-Distortion Metal Joining
- What is the process of a vacuum furnace? Achieve Purity and Precision in High-Temp Processing
- What are the factors that affect the strength of a brazed joint? Master the 4 Keys to a Perfect Bond
- Why would you braze instead of solder? For Superior Joint Strength and High-Temperature Performance
- What is the basic of brazing? A Guide to Strong, Low-Heat Metal Joining