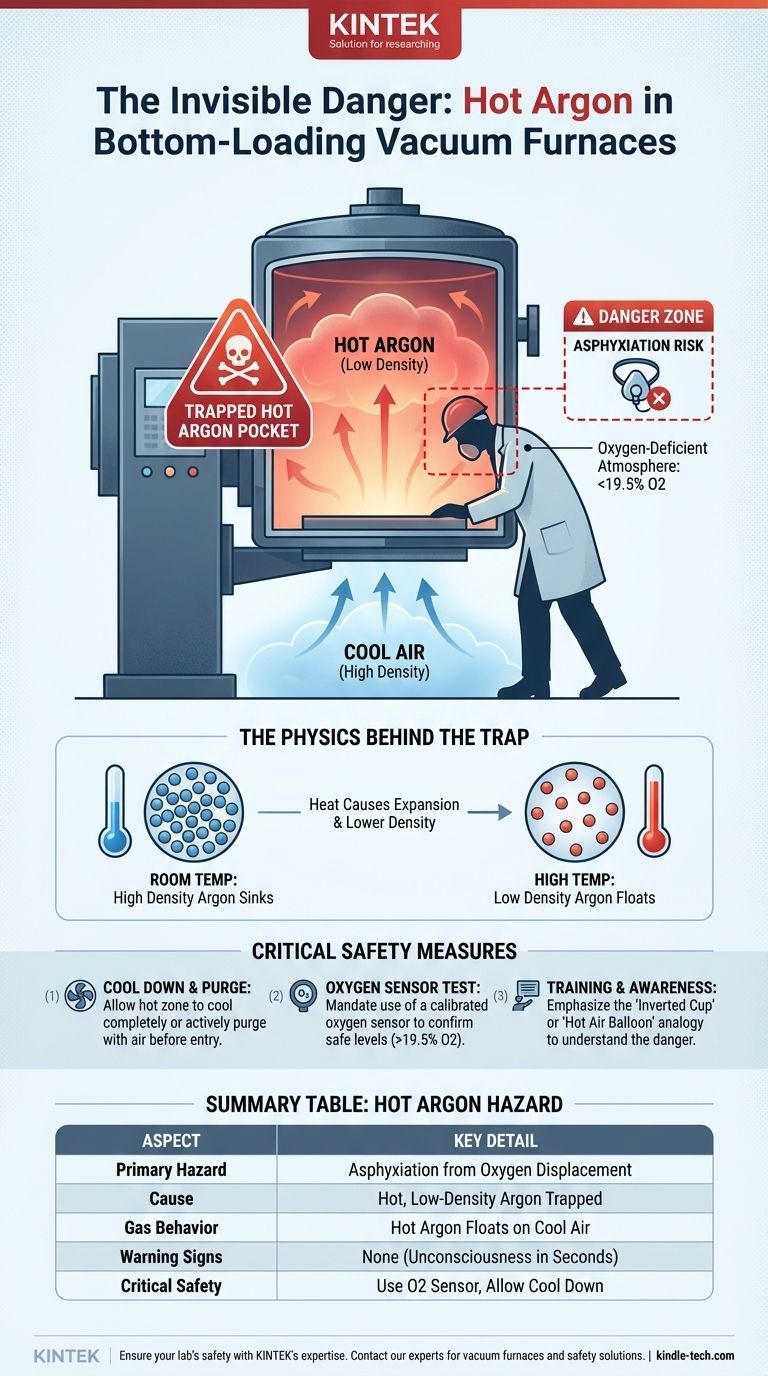
The specific danger of hot argon in a bottom-loading vacuum furnace is asphyxiation. This occurs because the hot, low-density argon gas does not immediately exit the open bottom of the furnace chamber. Instead, it remains trapped as an invisible, oxygen-displacing pocket, creating a lethal hazard for any operator who puts their head inside.
The core danger stems from a counter-intuitive principle: hot argon is less dense than cool air, causing it to "float" and become trapped inside the furnace chamber. This creates an invisible, oxygen-deficient zone precisely where an operator might lean in after a cycle, leading to a risk of sudden asphyxiation.

The Physics Behind the Hidden Danger
To fully appreciate the risk, you must first understand the physical principles at play within the furnace environment. The danger is not a result of a malfunction but is an inherent property of the system's normal operation.
The Principle of Gas Density
All gases change density with temperature. When a gas is heated, its molecules move faster and spread farther apart, making it less dense.
Conversely, as a gas cools, its molecules slow down and move closer together, making it more dense. This fundamental behavior is the root cause of the hazard.
How Hot Argon Becomes a "Trap"
Argon at room temperature is about 38% denser than air and would normally sink and flow out of an open-bottom container. However, when the furnace hot zone is still at a high temperature, the argon inside is also hot.
This hot, low-density argon behaves like a hot air balloon. It "floats" on the cooler, denser ambient air that enters the workspace from below. The furnace chamber effectively acts as an inverted cup, holding this buoyant pocket of argon in place.
The Invisible Hazard: Oxygen Displacement
Argon is an inert gas, meaning it is not toxic or poisonous. The danger it presents is its ability to displace oxygen. Normal air contains about 21% oxygen, which is essential for life.
When an operator puts their head into this trapped pocket of argon, they are entering an oxygen-deficient atmosphere. Unconsciousness can occur in seconds without any warning signs like choking or gasping, as there is nothing to irritate the respiratory system.
A Critical Safety Blind Spot
The nature of this hazard creates a significant blind spot in routine safety assumptions. The situation feels safe even when it is imminently deadly.
Counter-Intuitive Behavior
An operator's intuition suggests that with the bottom of the furnace open, any gas inside should have safely dissipated. The fact that the hot gas is trapped above the opening is a non-obvious and deeply counter-intuitive phenomenon.
False Sense of Security
The furnace cycle is complete, the hearth is lowered, and the chamber is open to the room. These cues signal the end of the high-risk portion of the process, which can lull an operator into a false sense of security while the invisible thermal and atmospheric hazard persists.
Making the Right Choice for Your Goal
Understanding this principle is the key to implementing effective safety protocols that account for the hidden reality of the environment.
- If your primary focus is operational safety: Never permit anyone to break the plane of the furnace opening until the hot zone has cooled sufficiently to eliminate the density difference or has been actively purged with air.
- If your primary focus is procedural development: Mandate the use of a calibrated oxygen sensor to test the atmosphere within the chamber opening before any physical entry is permitted for inspection or maintenance.
- If your primary focus is training: Emphasize the "inverted cup" or "hot air balloon" analogy to ensure all personnel understand why the danger exists, making them more likely to respect the protocol.
True workplace safety is achieved not by just following rules, but by understanding the physical principles that make those rules necessary.
Summary Table:
| Danger Aspect | Key Detail |
|---|---|
| Primary Hazard | Asphyxiation from oxygen displacement |
| Cause | Hot, low-density argon trapped in the furnace chamber |
| Gas Behavior | Hot argon is less dense than cool air, causing it to 'float' |
| Warning Signs | None; unconsciousness can occur in seconds |
| Critical Safety Measure | Use an oxygen sensor before entry; allow hot zone to cool |
Ensure your lab's safety with KINTEK's expertise. Our vacuum furnaces and safety solutions are designed with operator protection in mind. We provide the equipment and guidance to help you mitigate risks like hot argon hazards. Don't compromise on safety—contact our experts today to discuss how we can support your laboratory's specific needs with reliable, safe, and efficient lab equipment.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- Vacuum Heat Treat Sintering Brazing Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Molybdenum Vacuum Heat Treat Furnace
People Also Ask
- What is the leak rate for a vacuum furnace? Ensure Process Purity and Repeatability
- What is a vacuum furnace? The Ultimate Guide to Contamination-Free Thermal Processing
- What are the advantages of vacuum hardening? Achieve Superior Precision and Cleanliness for Critical Components
- What is the maximum temperature in a vacuum furnace? It Depends on Your Materials and Process Needs
- What materials are used in a vacuum furnace? Selecting the Right Hot Zone for Your Process



















