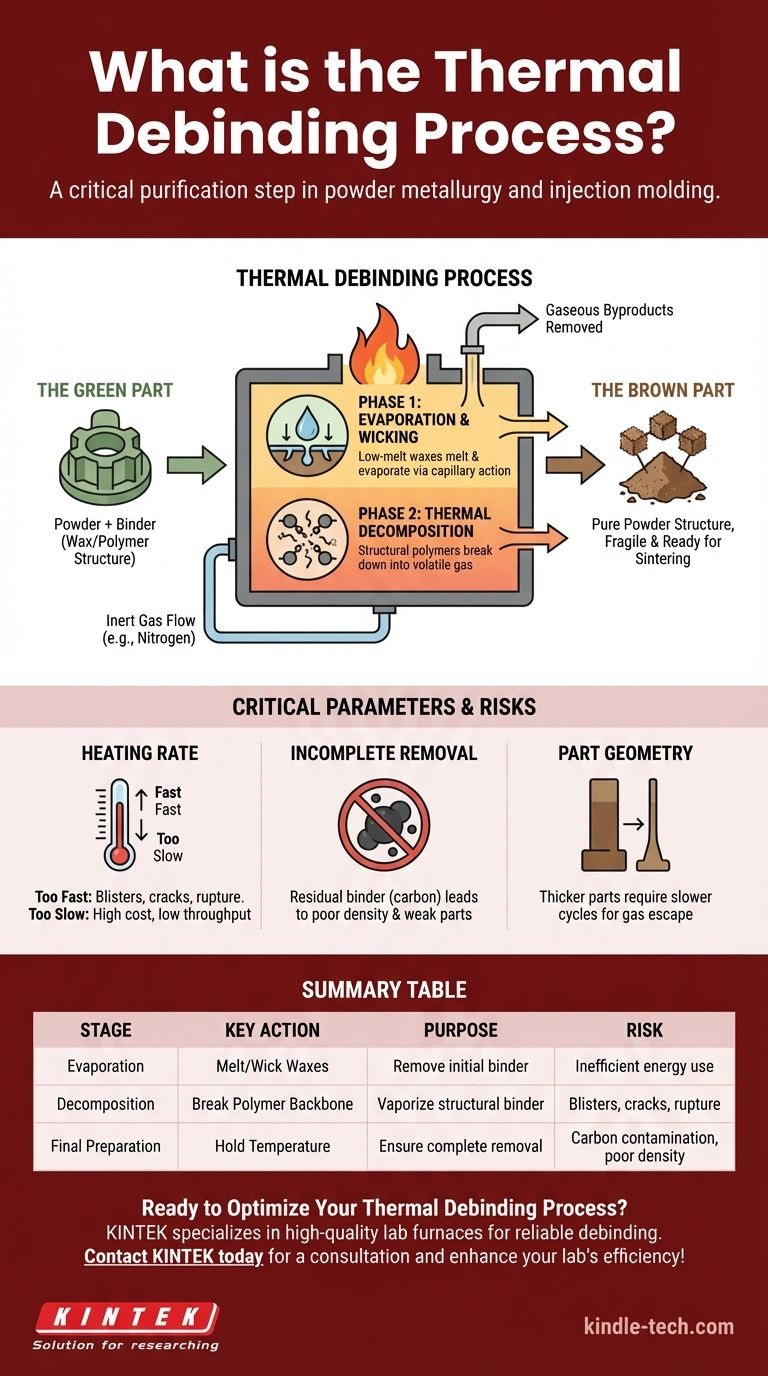
In essence, thermal debinding is a critical purification step used in powder metallurgy and injection molding. It is a highly controlled heating process designed to carefully remove a temporary polymer or wax "binder" from a molded component, known as a "green part." This is accomplished by heating the part in a furnace, causing the binder to either evaporate or chemically break down into a gas, which is then safely extracted.
Before a complex part made from metal or ceramic powder can be finalized, the sacrificial binder holding its shape must be removed. Thermal debinding is the crucial process that accomplishes this, transforming the part from a polymer-powder composite into a fragile, pure powder structure ready for final densification.

The Journey from "Green" to "Brown" Part
To understand thermal debinding, you must first understand why it's necessary. The entire process centers on the transition of the component through distinct stages.
The Role of the Binder in the "Green Part"
In manufacturing methods like Metal Injection Molding (MIM), a fine metal or ceramic powder is mixed with a binder system, typically composed of waxes and polymers. This mixture behaves like plastic, allowing it to be injected into a mold to form a complex, precisely shaped component. This initial, molded part is called the green part.
Why the Binder Must Be Removed
The binder is only a temporary scaffold. It provides no structural value to the final product and must be completely removed before the final step, known as sintering.
If binder remains during sintering (heating near the material's melting point), it will decompose uncontrollably, releasing gases that cause blisters, cracks, high porosity, and severe contamination in the final part.
Creating the Fragile "Brown Part"
After the binder has been successfully removed through debinding, the component is referred to as a brown part.
The brown part is extremely fragile, as it consists only of the primary powder particles held together by faint intermolecular forces. It must be handled with extreme care before it moves to the sintering furnace to be fused into a solid, dense object.
Unpacking the Mechanisms of Thermal Debinding
Thermal debinding is not simply a matter of melting the binder away. It's a sophisticated, multi-stage process governed by heat transfer, chemical reactions, and mass transport.
Phase 1: Evaporation and Wicking
The process begins at lower temperatures. The lower-melting-point components of the binder system, often waxes, melt into a liquid.
Through capillary action, this liquid binder "wicks" its way to the surface of the part, where it evaporates and is carried away by the furnace's atmosphere.
Phase 2: Thermal Decomposition
As the temperature increases, the structural "backbone" of the binder—typically a stronger polymer—begins to break down. This is not melting; it is thermal degradation.
The long polymer chains are chemically broken apart into smaller, volatile molecules (monomers and oligomers). These smaller molecules turn into a gas and diffuse out of the part.
The Critical Role of Furnace Atmosphere
The process occurs in a furnace with a tightly controlled atmosphere. An inert gas (like nitrogen or argon) is often used to flow through the furnace.
This gas flow is critical for two reasons: it prevents the metal powder from oxidizing at high temperatures, and it actively sweeps away the gaseous binder byproducts, preventing them from becoming trapped within the part.
Understanding the Trade-offs and Critical Parameters
The success of thermal debinding hinges on a delicate balance. Mismanaging the process parameters is a common source of defects.
The Danger of Heating Too Quickly
If the heating rate is too aggressive, the binder will vaporize deep inside the part faster than it can escape. This creates immense internal pressure.
The result is defects ranging from surface blisters and cracks to the complete rupture of the component. This is the single most common failure mode in thermal debinding.
The Cost of Heating Too Slowly
Conversely, an overly conservative, slow heating cycle ensures part safety but comes at a significant cost. It dramatically increases furnace time, reduces production throughput, and consumes far more energy.
The Problem of Incomplete Binder Removal
Failing to hold the part at the correct temperature for a sufficient duration can leave residual binder, often in the form of carbon, trapped within the powder structure.
This contamination will interfere with the atomic diffusion that occurs during sintering, leading to poor density and severely compromised mechanical properties in the final part.
The Impact of Part Geometry
Thicker or larger parts are significantly more challenging to debind. The binder byproducts have a much longer diffusion path to travel to escape the part's core. This necessitates much slower, more carefully controlled heating cycles to avoid internal pressure buildup.
How to Apply This to Your Process
Choosing the right debinding strategy requires aligning the process parameters with your primary manufacturing goal.
- If your primary focus is speed and throughput: Your goal is to find the fastest possible heating rate that does not introduce defects, supported by a high gas flow to efficiently remove byproducts.
- If your primary focus is part integrity: You must prioritize a slower, more controlled heating cycle, especially for thick cross-sections, to guarantee pressure does not build up and cause cracking.
- If your primary focus is material purity for critical applications: Emphasize using a high-purity furnace atmosphere and add verification steps to ensure no residual carbon is left behind before sintering.
Ultimately, mastering thermal debinding is about balancing the competing demands of speed, safety, and final part quality.
Summary Table:
| Stage | Key Action | Purpose | Risk if Mismanaged |
|---|---|---|---|
| Phase 1: Evaporation | Heat part to melt/wick low-melt binders (waxes). | Remove initial binder components via capillary action. | Slow production, inefficient energy use. |
| Phase 2: Decomposition | Increase temperature to break down polymer backbone. | Vaporize structural binder via thermal degradation. | Blisters, cracks, or part rupture from internal pressure. |
| Final Preparation | Hold temperature to ensure complete binder removal. | Create a pure, fragile "brown part" ready for sintering. | Carbon contamination, leading to poor density and weak final parts. |
Ready to Optimize Your Thermal Debinding Process?
Achieving the perfect balance between speed, part integrity, and final quality requires precise control. KINTEK specializes in high-quality lab furnaces and consumables designed for reliable thermal debinding, helping you prevent defects and ensure material purity.
Let our experts help you select the right equipment for your MIM, ceramic, or powder metallurgy needs. Contact KINTEK today for a consultation and enhance your lab's efficiency!
Visual Guide
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- How does a constant temperature reciprocating shaker influence adsorption kinetics? Optimize Your Pollutant Studies
- What is the rate of pyrolysis? A Key Variable for Controlling Bio-char, Bio-oil, and Syngas Yields
- What are the precautions to be taken while sampling? Ensure Data Accuracy and Minimize Bias
- What is the temperature of sintering ceramics? Mastering the Heat for Optimal Density and Strength
- Does tensile strength increase with heat treatment? How to Engineer the Perfect Metal Properties
- Why is precise temperature-controlled heating equipment required for chitosan synthesis? Ensure High-Quality Deacetylation
- How is temperature tracking managed in Ultra Freezers? From Basic Charts to Smart Alerts
- What is the primary purpose of using an ultrasonic cleaner for Pd/G-SS electrodes? Ensure Superior Coating Adhesion



















