Arc Melting
Concept and Classification
Electric arc furnace melting represents an advanced electrothermal metallurgical technique, leveraging electrical energy to generate an electric arc that serves as the primary heat source. This method is pivotal in the laboratory setting for its precision and control over the melting process. The classification of electric arc furnace melting primarily revolves around two distinct heating methods: direct and indirect heating.
In direct heating, the electric arc is established between the electrode and the metal charge, effectively transferring the electrical energy into thermal energy that directly heats the material. This method is further subdivided into non-vacuum direct heating and vacuum self-consumption arc melting, each tailored to specific metallurgical requirements and material properties.
On the other hand, indirect heating employs an electric arc generated between two graphite electrodes, which indirectly heats the metal charge. This approach is particularly advantageous for materials that require a more controlled and uniform heat distribution, minimizing the risk of contamination and ensuring higher purity in the final product.
The choice between direct and indirect heating methods is influenced by various factors, including the type of metal being melted, the desired purity levels, and the specific laboratory conditions. Each method offers unique advantages and is suited to different metallurgical applications, highlighting the versatility and strategic importance of electric arc furnace melting in modern laboratory practices.
Direct Heating Arc Melting
In direct heating arc melting, the arc is generated between the electrode and the charge, directly heating the material to extremely high temperatures, often exceeding 3000℃. This method is characterized by its efficiency and the direct transfer of heat to the material, which accelerates the melting process. The primary components of an arc melting furnace include the electric welder, which functions as a large transformer, converting standard voltage (either 220V or 380V) into a low voltage but high current. This high current is then utilized to create an arc through the instantaneous short circuit of the positive and negative poles.
The arc formed is a self-sustaining discharge phenomenon, capable of maintaining a stable combustion without the need for a high voltage to keep it from extinguishing. This stability is crucial for consistent and efficient melting. When shielded by electrodes, the arc's voltage can be increased to expedite the melting process, although this also introduces the risk of oxidation of elements like carbon, silicon, and manganese.
For large-scale arc production, a lower current is typically required, which minimizes heat loss and optimizes energy efficiency. The process can be further enhanced by deep bathing of the electrodes, ensuring a more thorough and rapid melting of the charge. This method is versatile, applicable both in non-vacuum environments and under vacuum conditions for self-consumption arc melting, offering flexibility in experimental settings and industrial applications.
Indirect Heating Arc Melting
In indirect heating arc melting, the process involves generating an electric arc between two graphite electrodes. Unlike direct heating methods where the arc directly contacts the charge, indirect heating relies on the transfer of heat from the arc to the charge through radiation. This configuration is often referred to as an indirect electric arc furnace.
The electric arc, formed between the two electrodes, does not come into direct contact with the material to be melted. Instead, the heat is primarily transferred to the top surface of the charge via radiation. This radiated heat then conducts through the charge, gradually melting it from the top down. This method ensures that the charge is heated evenly, although the efficiency of heat transfer can be lower compared to direct heating methods.
The use of two electrodes in indirect heating provides a controlled environment, which can be beneficial for certain materials that are sensitive to direct contact or rapid heating. This setup also allows for better control over the melting process, making it suitable for applications where precise temperature control and uniformity in the melt are critical.
| Aspect | Details |
|---|---|
| Electrode Configuration | Two graphite electrodes are used to generate the arc. |
| Heat Transfer Mechanism | Heat is transferred through radiation from the arc to the charge's surface. |
| Advantages | Provides controlled heating, suitable for sensitive materials. |
| Disadvantages | Lower efficiency in heat transfer compared to direct heating methods. |
Indirect heating arc melting is particularly advantageous in scenarios where maintaining the integrity and purity of the material is paramount, such as in the melting of certain alloys or reactive metals.
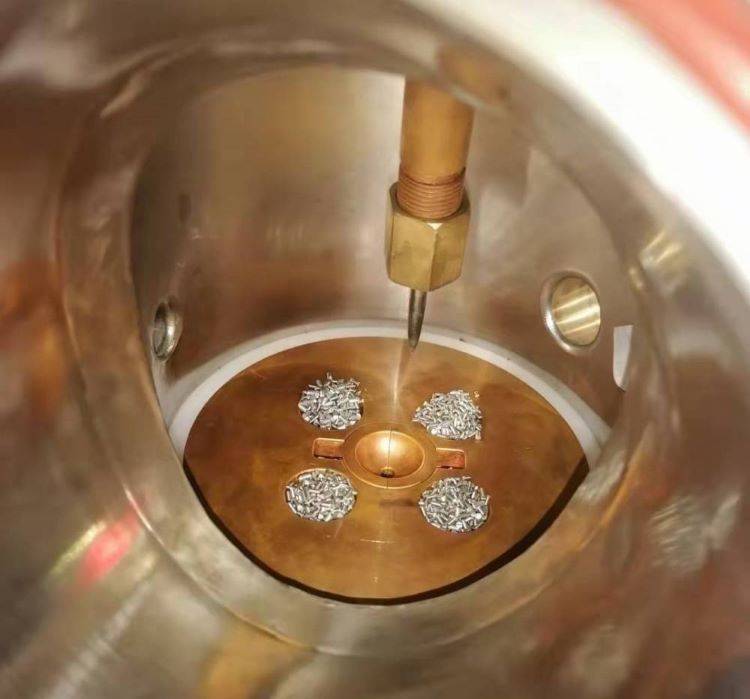
Melting Sequence
The melting sequence in laboratory settings is a meticulous process that requires careful consideration of several critical factors. These factors include the melting characteristics of refractory metals, the oxidizability of the materials, the potential for evaporation, and the density of the substances involved.
Refractory Metal Melting
Refractory metals, such as tungsten and molybdenum, pose unique challenges due to their high melting points. The melting process must ensure that the temperature is precisely controlled to avoid either underheating or overheating, which can compromise the integrity of the material.
Oxidizability
The oxidizability of the materials being melted is another crucial factor. Some metals are highly reactive with oxygen, which can lead to the formation of oxides that can contaminate the melt. Techniques such as vacuum melting are often employed to minimize oxidation and maintain the purity of the final product.
Evaporation
Evaporation during the melting process can result in significant material loss and can also affect the composition of the melt. To mitigate this, methods like vacuum or inert gas environments are used to create conditions that reduce the rate of evaporation.
Density
The density of the materials being melted can influence the flow and mixing of the melt. For instance, in induction melting, the electromagnetic stirring effect can be adjusted based on the density of the materials to ensure homogeneity and prevent segregation.
In summary, the melting sequence is a complex process that requires a thorough understanding and careful management of these factors to achieve successful and high-quality results.
Induction Melting
Concept and Principles
Vacuum Induction Melting (VIM) employs electromagnetic induction to heat the charge under vacuum conditions. This technique leverages the principles of induction heating and the controlled environment of a vacuum to achieve precise melting.
-
Induction Heating: The process involves the generation of an alternating magnetic field within a coil, which induces eddy currents in the conductive material to be melted. These currents generate heat directly within the material, leading to its melting. This method ensures efficient and localized heating, minimizing energy loss and enhancing control over the melting process.
-
Vacuum Environment: Operating under vacuum conditions is crucial for VIM. A vacuum environment eliminates atmospheric gases, which can cause oxidation and contamination. This is particularly beneficial for melting reactive metals and alloys, as it ensures high purity and prevents unwanted chemical reactions. The vacuum also helps in reducing the boiling point of materials, facilitating the melting of metals with high vapor pressures.
The combination of these principles allows VIM to achieve high-quality melts with minimal contamination, making it a preferred method for producing high-purity alloys and specialty metals.
Characteristics of Induction Melting
Induction melting boasts several distinctive features that set it apart from other melting methods. Electromagnetic induction heating is a cornerstone of this technique, where an alternating current flowing through a coil generates a magnetic field that induces currents within the metal charge, thereby heating it. This method ensures efficient and localized heating, minimizing energy loss and enhancing control over the melting process.
Another notable characteristic is electromagnetic stirring. This phenomenon occurs as the induced currents in the molten metal create their own magnetic fields, leading to a circulating motion within the melt pool. This stirring action is beneficial for promoting homogeneity in the alloy composition, aiding in the even distribution of elements and the removal of impurities.
The small melt pool surface area is another feature that contributes to the efficiency and cleanliness of induction melting. By containing the molten metal in a confined space, the process reduces the exposure of the melt to the atmosphere, thereby minimizing oxidation and other forms of contamination. This aspect is particularly advantageous in the production of high-purity alloys and reactive metals.
Lastly, induction melting is characterized by its environmental friendliness. The process generates less pollution compared to traditional melting methods, as it operates under controlled conditions and with minimal emissions. The use of advanced induction furnaces, equipped with solid-state IGBT frequency conversion and comprehensive protection mechanisms, further enhances the environmental sustainability of this technique. These furnaces are designed with features such as overcurrent protection, water shortage protection, overheating protection, and more, ensuring not only the reliability of the equipment but also its compliance with environmental standards.

Process Stages
The induction melting process is a meticulously orchestrated sequence of stages, each playing a crucial role in ensuring the quality and purity of the final product. The process can be broken down into four primary stages: charging, melting, refining, and pouring.
-
Charging: This initial stage involves loading the raw materials, or charge, into the induction furnace. The choice of charge materials is critical, as it directly impacts the composition and quality of the molten metal. The materials are usually pre-weighed and pre-mixed to ensure consistency.
-
Melting: Once the charge is in place, the induction coils generate a magnetic field that induces currents within the charge, causing it to heat up and melt. This stage is characterized by electromagnetic stirring, which helps to homogenize the molten metal and promote uniform heating.
-
Refining: After the metal has melted, it undergoes a refining process to remove impurities. This can involve various techniques such as deoxidation, desulfurization, and degassing. The vacuum environment of the induction furnace aids in this process by minimizing the presence of atmospheric gases that could introduce impurities.
-
Pouring: The final stage involves pouring the refined molten metal into molds or ingot molds. The pouring process must be carefully controlled to avoid contamination and ensure the metal solidifies correctly. The choice of pouring temperature and speed is critical to prevent defects such as porosity or shrinkage.
Each of these stages is meticulously managed to ensure the final product meets the required specifications for purity, composition, and mechanical properties.
Crucible Selection
Selecting the right crucible is a meticulous process that requires careful consideration of various factors to ensure both contamination prevention and thermal stability. The modern crucible is a sophisticated composite material, primarily composed of graphite, which leverages its unique structural alignment and material composition to meet the stringent performance requirements demanded by different applications.
Crucibles come in an extensive array of sizes, shapes, and configurations, catering to a wide range of industrial needs. They can be as diminutive as teacups or as capacious as containers capable of holding several tons of metal. Their design versatility allows them to be either fixed within a furnace structure or engineered for easy removal for pouring operations. Crucibles are integral to various furnace types, including fuel-fired, electric resistance, and induction furnaces, and they are often equipped with or without pouring spouts, depending on the specific operational requirements.

The selection process is further complicated by the multitude of performance characteristics that crucibles exhibit. Each application presents a unique set of temperature, chemical, and physical parameters that define the operational boundaries within which the crucible must function effectively. For instance, the crucible used in an induction furnace must withstand electromagnetic forces, while those in fuel-fired furnaces need to endure direct thermal exposure.
Given the extensive range of crucible types and materials available, choosing the optimal crucible for a specific operation is a complex and individualized task. The selection process should be tailored to the unique combination of furnaces, alloys, metallurgical treatments, and pouring arrangements employed in each facility. This customization ensures that the crucible provides maximum performance, thereby optimizing the overall efficiency and reliability of the metal melting process.
In summary, the selection of a crucible is not merely a matter of choosing a container for molten metal; it is a critical decision that impacts the quality, safety, and efficiency of the entire melting operation. Therefore, it is imperative that metal melters and crucible suppliers collaborate closely to ensure the chosen crucible meets all the specific requirements of the application.
Suspension Melting
Concept and Advantages
Suspension melting technology represents a significant advancement in laboratory melting methods, particularly for achieving high purity and homogeneity in molten materials. This technique involves maintaining the molten material in a state of suspension or quasi-suspension, which is a critical factor in preventing crucible contamination. By eliminating direct contact between the molten material and the crucible, suspension melting significantly reduces the risk of impurities entering the melt.
This method is particularly advantageous for materials that are highly sensitive to contamination, such as active metals, high purity metals, precise alloys, and high melting point metals. The absence of a traditional crucible not only enhances purity but also allows for better control over the melting process, leading to more uniform and consistent results. The ability to maintain the molten material in a suspended state also facilitates better heat distribution and reduces the likelihood of localized overheating, which can otherwise lead to defects or inconsistencies in the final product.
In summary, suspension melting offers a unique set of benefits that make it an invaluable tool in laboratory settings, particularly for applications requiring the utmost purity and homogeneity in the final product.
Full Suspension Induction Melting
Full Suspension Induction Melting is a sophisticated technique that involves the melting of materials in a completely suspended state without the use of a traditional crucible. This method leverages the principles of electromagnetic induction to create a magnetic field that suspends and heats the material simultaneously. The absence of a crucible eliminates the risk of contamination from crucible materials, thereby ensuring the highest purity and homogeneity of the molten metal.
This technique is particularly advantageous for melting high-purity metals and alloys, as well as reactive metals that are susceptible to contamination. The electromagnetic field not only suspends the material but also induces a stirring effect, which promotes uniform heating and prevents localized overheating. This results in a more controlled and homogeneous melt, which is crucial for applications requiring precise chemical compositions.
The process typically involves several stages: initial charging of the material into the induction coil, followed by the application of the electromagnetic field to induce melting, and finally, the controlled pouring of the molten material. The entire process is conducted under a vacuum or inert gas environment to prevent oxidation and other atmospheric reactions.
Full Suspension Induction Melting represents a significant advancement in metallurgical techniques, offering unparalleled control and purity in the melting process. Its applications extend to various industries, including aerospace, electronics, and specialty alloys, where high purity and precise control are paramount.
Cold Crucible Induction Melting
Cold Crucible Induction Melting (CCIM) is a sophisticated technique that employs a water-cooled copper crucible within an alternating electromagnetic field to melt metals. This method is particularly advantageous for its ability to prevent contamination, which is crucial for high-purity materials.
The water-cooled copper crucible, often referred to as the "cold crucible," is designed to remain cool despite the intense heat generated by the electromagnetic field. This cooling mechanism ensures that the crucible itself does not become a source of contamination, which is a significant concern in traditional crucible melting methods.

The alternating electromagnetic field is generated by induction coils surrounding the crucible. This field induces eddy currents within the metal charge, which in turn generates heat through resistance. The process is highly efficient and allows for precise control over the melting conditions, making it ideal for materials that require careful handling to maintain purity and homogeneity.
One of the key benefits of CCIM is its ability to melt reactive and high-purity metals without introducing impurities from the crucible material. This makes it particularly useful in applications where contamination is a critical issue, such as in the production of advanced alloys and materials for aerospace and electronics industries.
In summary, Cold Crucible Induction Melting leverages advanced technology to provide a contamination-free melting environment, making it an indispensable tool in the quest for high-purity metals and alloys.
Applications
Suspension melting technology is particularly advantageous in several specialized applications due to its unique method of keeping the molten material in a suspension or quasi-suspension state. This method effectively eliminates crucible contamination, thereby ensuring the attainment of high purity and homogeneity in the final product.
One of the primary applications of suspension melting is in the processing of active metals. These metals, which are highly reactive with oxygen and other atmospheric gases, require an environment where they can be melted without exposure to contaminants. Suspension melting provides such an environment, making it an ideal choice for metals like titanium and zirconium.
Another significant application is in the production of high purity metals. The absence of crucible materials in suspension melting means that there is no risk of leaching impurities into the melt, which is crucial for applications where even trace amounts of contamination can be detrimental. This makes it suitable for metals used in semiconductor manufacturing and other high-tech industries.
Suspension melting is also employed in the creation of precise alloys. The controlled environment and the ability to maintain a homogeneous melt without external contamination allow for the precise control of alloy composition, which is essential for creating alloys with specific mechanical and chemical properties.
Lastly, this technique is invaluable for high melting point metals. The ability to melt these metals without the need for a traditional crucible, which might not withstand the high temperatures required, ensures that the melting process can be conducted efficiently and safely. Metals such as tungsten and tantalum, which have extremely high melting points, benefit greatly from this method.
In summary, suspension melting is a versatile and powerful technique, particularly suited for materials that require high purity, precise control, and the ability to withstand extreme temperatures.
Related Products
- Vacuum Arc Induction Melting Furnace
- Non Consumable Vacuum Arc Induction Melting Furnace
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- Vacuum Induction Melting Spinning System Arc Melting Furnace
- Cylindrical Lab Electric Heating Press Mold for Laboratory Applications
Related Articles
- Vacuum Melting Furnace: A Comprehensive Guide to Vacuum Induction Melting
- Exploring Tungsten Vacuum Furnaces: Operation, Applications, and Advantages
- Melting process and maintenance of vacuum induction melting furnace
- Vacuum Induction Melting Furnace: Principle, Advantages, and Applications
- Vacuum Laboratory Furnaces in Advanced Materials Research























