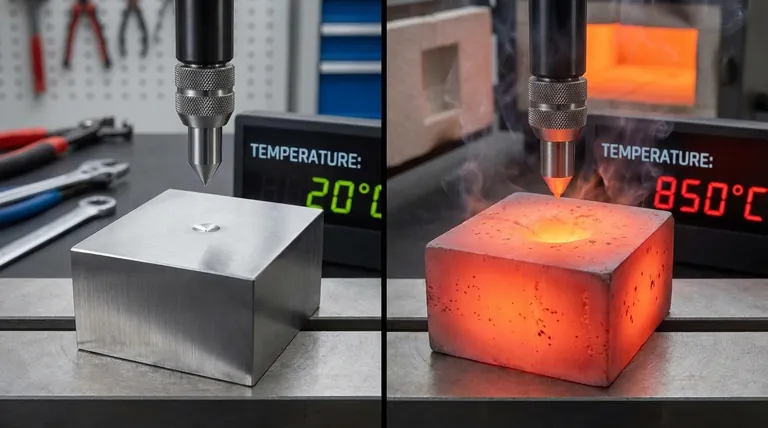
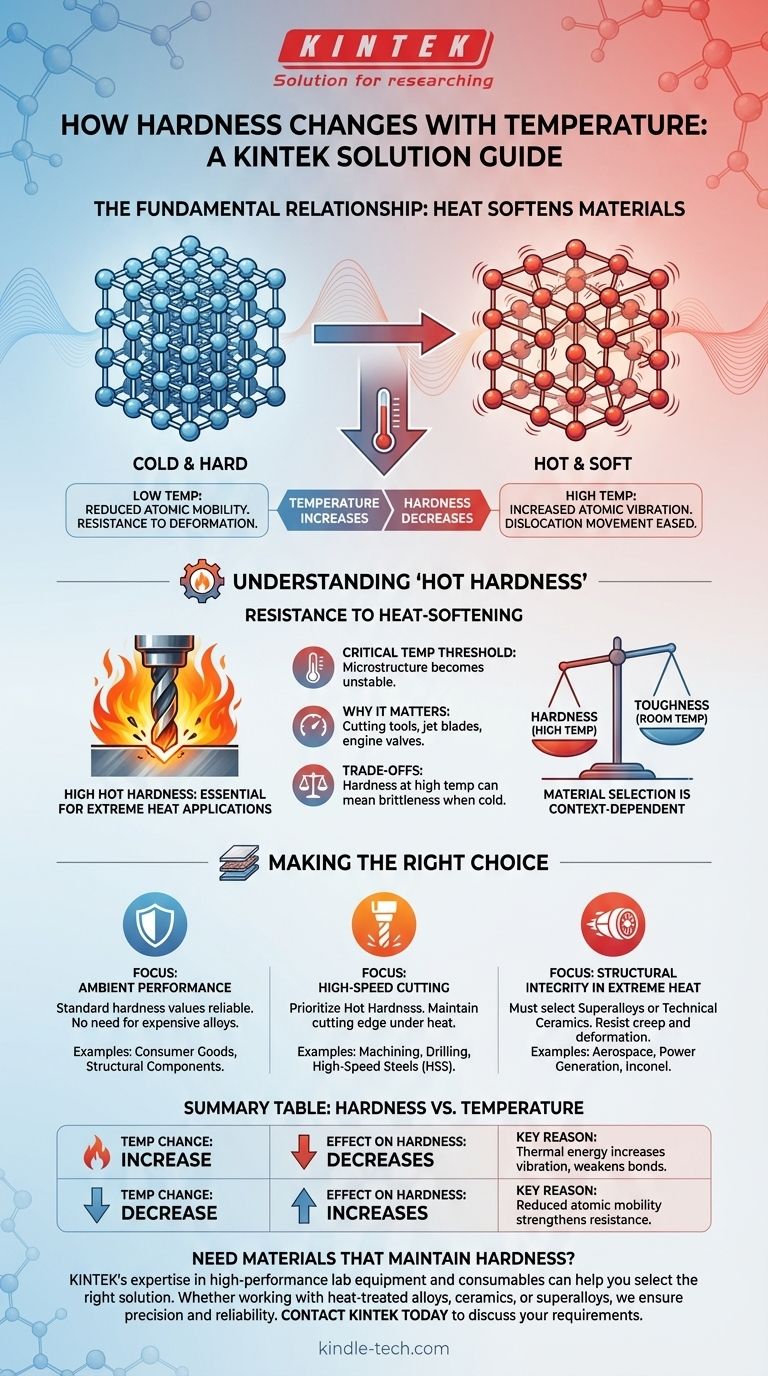
In nearly all materials, hardness has an inverse relationship with temperature. As the temperature of a material increases, its hardness decreases. This happens because thermal energy makes the material's internal structure easier to deform under pressure.
The core principle is that heat provides energy that allows atoms within a material's crystal lattice to move more easily. This increased atomic mobility reduces the material's resistance to permanent deformation, which is what we measure as hardness.
The Fundamental Relationship: Why Heat Softens Materials
To understand why hardness drops with heat, we need to look at the atomic level. Hardness is a measure of a material's resistance to localized plastic deformation, such as a scratch or indentation.
Atomic Vibration and Bond Strength
Heat is a form of energy. When a material is heated, its atoms absorb this energy and begin to vibrate more intensely. This increased vibration effectively weakens the interatomic bonds that hold the crystal structure together.
Facilitating Dislocation Movement
The actual process of plastic deformation in crystalline materials is governed by the movement of defects called dislocations. For a material to deform, these dislocations must move through the crystal lattice. The added thermal energy from heating makes it significantly easier for these dislocations to overcome barriers and slip, resulting in a softer material.
Understanding "Hot Hardness"
While all materials get softer when heated, some are specifically designed to resist this effect. This property is critical in many high-performance engineering applications.
What Is Hot Hardness?
Hot hardness (sometimes called red hardness) is the ability of a material to retain its hardness at elevated temperatures. Materials with high hot hardness are essential for applications that generate significant heat.
The Critical Temperature Threshold
For any given material, there is a temperature at which its hardness begins to drop drastically. This is the point where the underlying microstructure becomes unstable. For tool steels, this is the temperature at which the cutting edge would dull almost instantly.
Why It Matters
This property is crucial for components like high-speed cutting tools, jet engine turbine blades, and automotive engine valves. These parts must remain hard and strong while operating at extreme temperatures to avoid rapid wear and failure.
The Trade-offs and Considerations
Designing for hot hardness involves balancing several competing material properties. It is not a universal solution for all applications.
Material Selection Is Context-Dependent
A material with exceptional hardness at room temperature may be entirely unsuitable for a high-temperature application. For example, a standard carbon steel file is very hard but will lose its hardness completely if heated to a dull red, whereas a high-speed steel drill bit will not.
The Role of Alloying
Materials like heat-treated alloys and superalloys achieve their hot hardness through the addition of specific elements. Elements like tungsten, molybdenum, cobalt, and chromium form stable, hard microscopic particles (carbides) within the steel that physically obstruct dislocation movement even at high temperatures.
Hardness vs. Brittleness
Often, materials engineered for extreme hot hardness can be more brittle at room temperature. The same microstructural features that block dislocations at high temperatures can make the material less able to absorb impacts without fracturing when cold.
Making the Right Choice for Your Goal
Understanding this relationship is fundamental to selecting the correct material for any application involving heat. Your choice depends entirely on the expected operating environment.
- If your primary focus is performance at ambient temperatures: Standard hardness values are a reliable guide, and you do not need to prioritize specialized and expensive high-temperature alloys.
- If your primary focus is high-speed cutting or machining: Prioritize materials with excellent hot hardness, such as high-speed steels (HSS), cobalt alloys, or ceramics, which maintain their cutting edge under intense friction and heat.
- If your primary focus is structural integrity in extreme heat: You must select superalloys (e.g., Inconel) or technical ceramics designed specifically to resist deformation and creep at their target service temperatures.
Ultimately, recognizing that hardness is a dynamic property dependent on temperature is the first step toward preventing mechanical failure in thermally demanding applications.
Summary Table:
| Temperature Change | Effect on Hardness | Key Reason |
|---|---|---|
| Increase | Decreases | Thermal energy increases atomic vibration, weakening bonds and easing dislocation movement. |
| Decrease | Increases | Reduced atomic mobility strengthens the material's resistance to deformation. |
Need materials that maintain hardness at high temperatures? KINTEK's expertise in high-performance lab equipment and consumables, including materials for thermal analysis and high-temperature processing, can help you select the right solution. Whether you're working with heat-treated alloys, ceramics, or superalloys, our team ensures your laboratory operates with precision and reliability. Contact KINTEL today to discuss your high-temperature application requirements.
Visual Guide
Related Products
- High-Purity Titanium Foil and Sheet for Industrial Applications
- Molybdenum Disilicide (MoSi2) Thermal Elements Electric Furnace Heating Element
- High Purity Zinc Foil for Battery Lab Applications
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Custom Machined and Molded PTFE Teflon Parts Manufacturer for Laboratory ITO FTO Conductive Glass Cleaning Flower Basket
People Also Ask
- How does catalytic pyrolysis work? Unlock Higher Yields of Valuable Fuels and Chemicals
- What are the advantages of using the Pechini sol-gel method? Boost Perovskite Quality with Molecular-Level Precision
- Why is it important to match the freezer temperature to storage recommendations? Optimize Food Safety & Energy Use
- How does rapid quenching equipment stabilize actinide elements? Mastering Advanced Nuclear Waste Treatment
- What is the difference between sintering and annealing? A Guide to Choosing the Right Thermal Process
- What are the precautions of annealing? Master the 4 Keys to Precise Heat Treatment
- What are the problems for bio oils utilization? Overcome Key Barriers to Renewable Fuel Adoption
- What role do thermostatic shakers and Erlenmeyer flasks play in bioconversion? Optimize Your Xylose to Xylitol Process



















