Carbon coating is a surface modification process where a thin layer of carbon is deposited onto the surface of another material, often a powder. The most common methods involve heating an organic precursor (like sugar or a polymer) with the host material in an inert atmosphere, a process known as pyrolysis, or using gas-phase techniques like chemical vapor deposition.
The goal of carbon coating is not simply to add a layer, but to engineer a conductive and protective interface on a material's surface. The specific technique chosen is always a function of the core material's properties and the performance bottleneck—such as poor conductivity or instability—that needs to be solved.

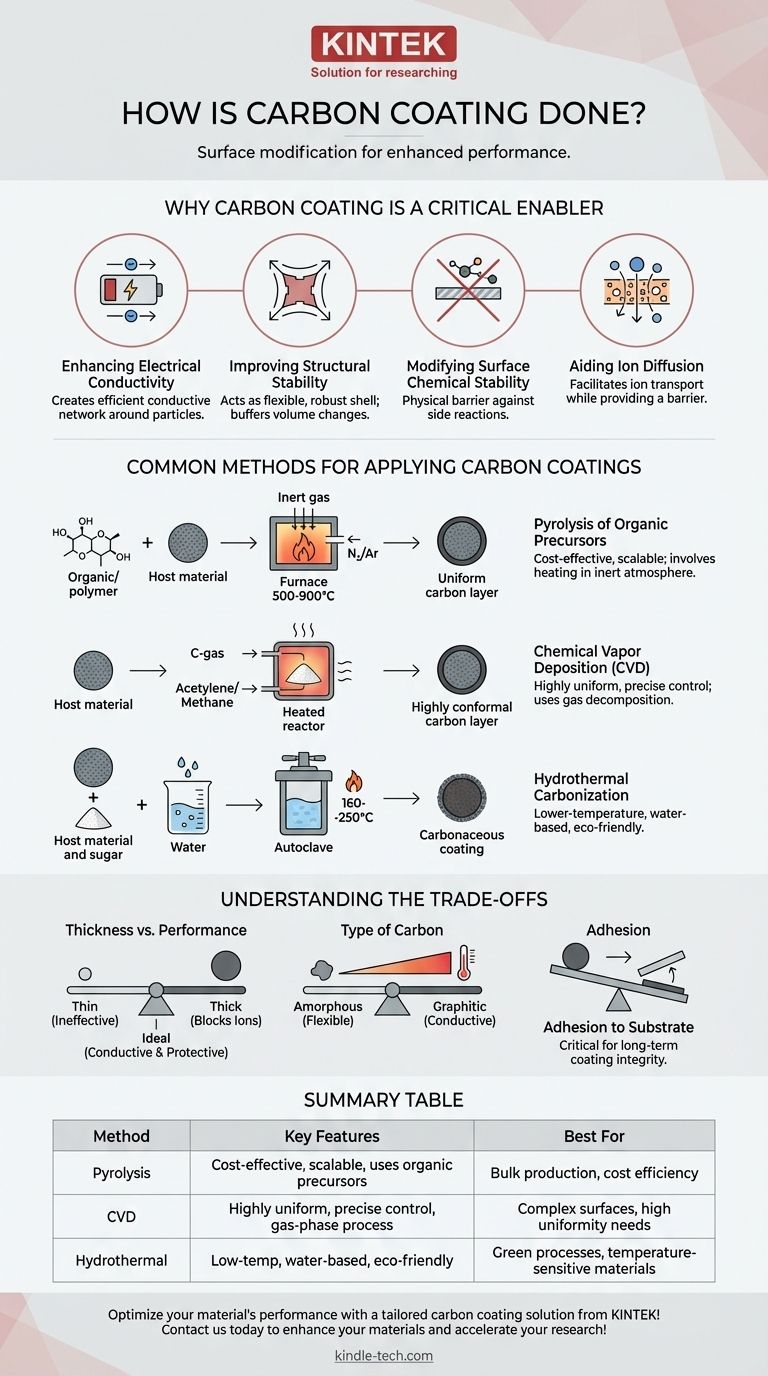
Why Carbon Coating is a Critical Enabler
Before examining the methods, it's essential to understand the problems carbon coating solves, particularly in high-performance materials like those used in batteries. The coating fundamentally alters the material's interaction with its environment.
Enhancing Electrical Conductivity
Many advanced electrode materials, especially for batteries, have poor intrinsic electrical conductivity.
A thin, uniform carbon layer creates an efficient conductive network around each particle, ensuring electrons can move easily to and from the material during operation.
Improving Structural Stability
Some materials, like silicon anodes, undergo significant volume changes during charging and discharging.
The carbon coating acts as a flexible, mechanically robust shell. It helps buffer this expansion and contraction, preventing the particle from cracking and losing electrical contact over time.
Modifying Surface Chemical Stability
Bare surfaces of reactive materials can undergo undesirable side reactions, for example, with the electrolyte in a battery.
This carbon layer serves as a physical barrier, preventing direct contact and passivating the surface. This dramatically reduces degradation and improves the material's cycle life and safety.
Aiding Ion Diffusion
While providing a barrier, a well-designed coating must still allow ions (like Li-ions) to pass through.
A properly structured carbon coating can be engineered to facilitate ion transport, ensuring the protective layer doesn't impede the core function of the material.
Common Methods for Applying Carbon Coatings
The method used to apply the coating is critical, as it determines the coating's thickness, uniformity, and the type of carbon produced.
Pyrolysis of Organic Precursors
This is the most common and scalable method. The core material is mixed with a carbon-containing organic compound, such as glucose, pitch, or various polymers.
The mixture is then heated to a high temperature (typically 500-900°C) in an inert atmosphere (like nitrogen or argon). The heat decomposes the organic precursor, leaving behind a carbon residue on the material's surface.
Chemical Vapor Deposition (CVD)
In CVD, the material to be coated is heated in a reactor. A carbon-containing gas, such as acetylene or methane, is then introduced.
At high temperatures, these gases decompose on the hot surface of the material, depositing a highly uniform and conformal layer of carbon. This method offers excellent control but is often more complex and expensive.
Hydrothermal Carbonization
This is a lower-temperature, water-based method. The material is suspended in water with a carbon source, typically a sugar like glucose.
The mixture is sealed in an autoclave and heated to around 160-250°C. The high pressure and temperature cause the sugar to dehydrate and form a carbonaceous coating on the particles.
Understanding the Trade-offs
Applying a carbon coating is not without its challenges. The effectiveness is dictated by a careful balance of several factors.
Coating Thickness vs. Performance
An ideal coating is just thick enough to provide conductivity and protection.
If the coating is too thin or non-uniform, it fails to do its job. If it's too thick, it can block ion pathways and add "dead weight," reducing the overall energy density of the material.
Type of Carbon
The temperature and method used determine the structure of the carbon. Lower temperatures often yield amorphous carbon, which is less conductive but more flexible.
Higher temperatures can produce more ordered, graphitic carbon, which offers superior electrical conductivity but can sometimes be more brittle.
Adhesion to the Substrate
The bond between the carbon layer and the core material is critical. If adhesion is poor, the coating can delaminate during processing or operation, rendering it completely useless. The choice of precursor and process conditions heavily influences this property.
Making the Right Choice for Your Goal
Selecting the correct carbon coating strategy depends entirely on your material, budget, and desired outcome.
- If your primary focus is cost-effective, bulk production: Pyrolysis of simple organic precursors is the most practical and widely used approach.
- If your primary focus is a highly precise and uniform coating on complex surfaces: Chemical Vapor Deposition (CVD) provides unparalleled control, albeit at a higher cost.
- If your primary focus is a green, lower-temperature process: Hydrothermal carbonization offers an effective, water-based alternative to high-temperature pyrolysis.
Ultimately, the most effective carbon coating is one that is meticulously engineered to solve the specific performance bottleneck of your core material.
Summary Table:
| Method | Key Features | Best For |
|---|---|---|
| Pyrolysis | Cost-effective, scalable, uses organic precursors | Bulk production, cost efficiency |
| Chemical Vapor Deposition (CVD) | Highly uniform, precise control, gas-phase process | Complex surfaces, high uniformity needs |
| Hydrothermal Carbonization | Low-temperature, water-based, eco-friendly | Green processes, temperature-sensitive materials |
Optimize your material's performance with a tailored carbon coating solution from KINTEK!
Our expertise in lab equipment and consumables ensures you get the right coating method—whether it's cost-effective pyrolysis, precise CVD, or eco-friendly hydrothermal carbonization—to solve your specific challenges in conductivity, stability, and longevity.
Contact us today to discuss how we can enhance your materials and accelerate your research!
Visual Guide
Related Products
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- How are optical coatings made? A Guide to Precision Thin-Film Deposition
- What is the process of CVD semiconductor? A Step-by-Step Guide to Thin Film Deposition
- What is the energy range of sputtering? From Threshold to Optimal Deposition
- What are the advantages of chemical vapour deposition method for synthesis of nanomaterials? Precision Engineering at the Nanoscale
- How are thin films made? A Guide to PVD, CVD, and ALD Deposition Techniques
- What is the mechanism of sputter deposition? A Step-by-Step Guide to Thin Film Coating
- What is the synthesis of CNT using CVD method? Grow High-Quality Carbon Nanotubes Efficiently
- What is material deposition in manufacturing? Unlock Design Freedom with Additive Processes



















