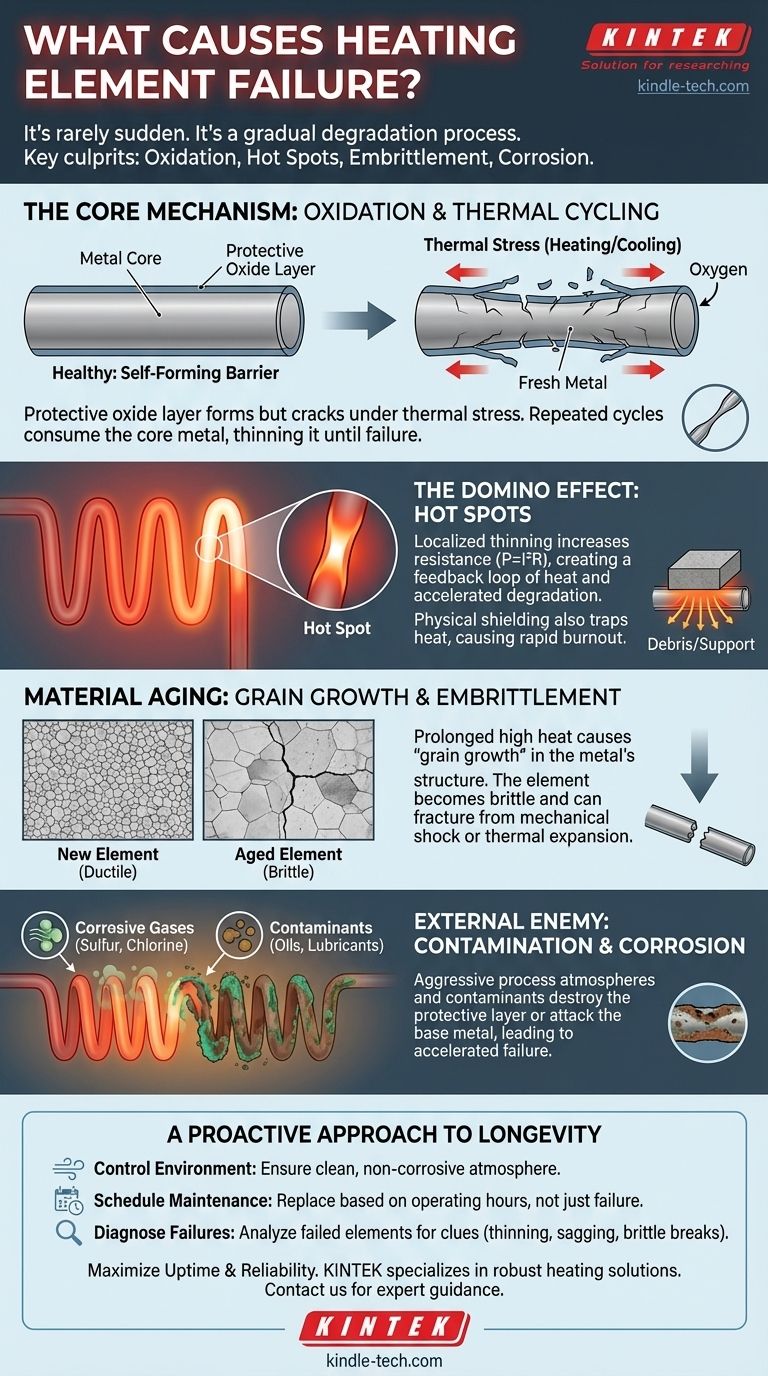
In nearly all cases, heating element failure is not a sudden event but the result of a gradual process of degradation. The primary causes are oxidation accelerated by thermal cycling, the formation of localized hot spots, material embrittlement from prolonged heat exposure, and chemical corrosion from the operating environment.
The core issue is that a heating element's own protective mechanisms are slowly broken down by the very conditions it creates. Understanding this inevitable process of aging is the key to maximizing its operational lifespan and preventing unexpected downtime.

The Core Mechanism: Oxidation and the Protective Layer
The most common heating elements, such as those made of nickel-chromium (Nichrome) or iron-chromium-aluminum (FeCrAl), are designed to operate at extreme temperatures. Their survival depends on a delicate chemical balance.
How a Healthy Element Protects Itself
When first heated, the element's surface reacts with oxygen in the air. This isn't a flaw; it's a feature. This reaction forms a thin, stable, and electrically non-conductive layer of oxide—typically chromium oxide or aluminum oxide.
This protective oxide layer acts like a skin, preventing oxygen from reaching the raw metal underneath. It's this self-forming barrier that allows the element to survive for thousands of hours at high temperatures.
The Vicious Cycle of Thermal Stress
The problem arises from thermal cycling—the process of heating up and cooling down. As the element heats, it expands. As it cools, it contracts.
The metal alloy and its protective oxide layer have different rates of thermal expansion. This mismatch creates immense mechanical stress, which can cause the brittle oxide layer to crack and flake off, exposing fresh, unprotected metal to the atmosphere.
When the element is heated again, a new oxide layer forms on this exposed area. This repeated process consumes the core material of the element, gradually thinning it out until it can no longer carry the electrical current and fails.
The Domino Effect: How Hot Spots Accelerate Failure
Uniform heating is critical to an element's health. A "hot spot" is any section of the element that operates at a significantly higher temperature than its surrounding areas, creating a localized point of rapid degradation.
What is a Hot Spot?
As an element thins due to oxidation or other damage, its electrical resistance increases in that specific spot. According to Ohm's law (Power = I²R), a higher resistance in one section forces it to dissipate more power as heat.
This creates a feedback loop: the spot gets hotter, which accelerates local oxidation, which thins the element further, which increases its resistance, which makes it even hotter. This cascade leads to a rapid burn-out at that specific point.
The Role of Physical Shielding
Hot spots are also commonly caused by anything that prevents the element from radiating its heat uniformly. This is often due to contact with refractory supports, insulation, or debris.
If a section of the element is "shielded," the heat it generates cannot escape. The temperature in that spot rises dramatically, initiating the same failure cascade described above.
Understanding the Trade-offs: Embrittlement and Material Aging
Even in a perfect environment with stable temperatures, an element has a finite life. This is due to internal changes in its metallic structure.
The Inevitable Process of Grain Growth
At a microscopic level, the metal in a heating element is composed of crystalline structures called "grains." When held at high temperatures for long periods, these small grains slowly merge and grow into larger ones.
This process of grain growth is an unavoidable consequence of prolonged heat exposure.
Why Brittleness Leads to Mechanical Failure
An element with large grains becomes very brittle, especially after it cools to room temperature. While it might function perfectly when hot, it loses all its ductility.
This is why old elements often fail not during operation, but during maintenance or on the next startup. The slightest mechanical shock or the stress of thermal expansion can be enough to cause the brittle material to fracture.
The External Enemy: Contamination and Corrosion
The atmosphere in which an element operates has a profound impact on its lifespan. Chemical reactions can destroy the protective oxide layer or attack the base metal directly.
The Threat from Process Atmospheres
Certain gases are highly corrosive to heating elements. For example, reducing atmospheres (like hydrogen or cracked ammonia) can prevent the formation of the protective oxide layer, leading to rapid failure.
Gases containing sulfur, chlorine, or other halogens are also extremely aggressive and will quickly corrode most common element alloys.
The Danger of Common Contaminants
Contaminants introduced into the furnace, such as oils, lubricants, or cleaning fluids, can carbonize on the element's surface. This can lead to carburization, which alters the alloy's properties, lowers its melting point, and often causes catastrophic failure.
A Proactive Approach to Element Longevity
Understanding these failure modes allows you to shift from a reactive to a proactive maintenance strategy. Your goal is to slow these inevitable processes.
- If your primary focus is extending life in a continuous-use process: Ensure the furnace atmosphere is clean and non-corrosive, and check that elements are not physically touching supports or debris.
- If your primary focus is reliability in a high-cycle environment: Select an element alloy known for its resistance to thermal cycling and consider a preventative replacement schedule based on operating hours.
- If you are diagnosing a recent failure: Examine the failed element for clues—thinning and green discoloration suggest oxidation (Nichrome), sagging or melting indicates a severe hot spot, and a clean, sharp break points to embrittlement.
By treating the heating element as a critical component whose environment you can control, you can significantly improve its reliability and the predictability of your operations.
Summary Table:
| Failure Cause | Key Mechanism | Impact on Element |
|---|---|---|
| Oxidation & Thermal Cycling | Mismatched expansion cracks the protective oxide layer, exposing fresh metal. | Gradual thinning and eventual burnout. |
| Hot Spot Formation | Localized high resistance creates a feedback loop of increasing heat. | Rapid, localized burn-out and failure. |
| Material Embrittlement | Prolonged heat causes grain growth, making the metal brittle. | Fractures during cooling or startup. |
| Chemical Corrosion | Aggressive atmospheres or contaminants destroy the oxide layer. | Accelerated corrosion and catastrophic failure. |
Maximize your lab's uptime and equipment reliability. The failure of a heating element can halt your critical processes. At KINTEK, we specialize in lab equipment and consumables, providing robust heating solutions and expert guidance to help you control the operating environment and extend the life of your vital components.
Let our experts help you select the right elements and implement a proactive maintenance strategy. Contact KINTEK today to ensure your laboratory operations run smoothly and predictably.
Visual Guide
Related Products
- Molybdenum Disilicide (MoSi2) Thermal Elements Electric Furnace Heating Element
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Vertical Laboratory Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- What are the properties of molybdenum heating element? Choose the Right Type for Your Furnace Atmosphere
- What is the temperature range of a MoSi2 heating element? Unlock 1900°C Performance for Your Lab
- Is molybdenum disulfide a heating element? Discover the best material for high-temperature applications.
- What function do molybdenum disilicide heating elements perform? Precision Heat for Pulverized Coal Research
- What material is used for furnace heating? Select the Right Element for Your Process



















