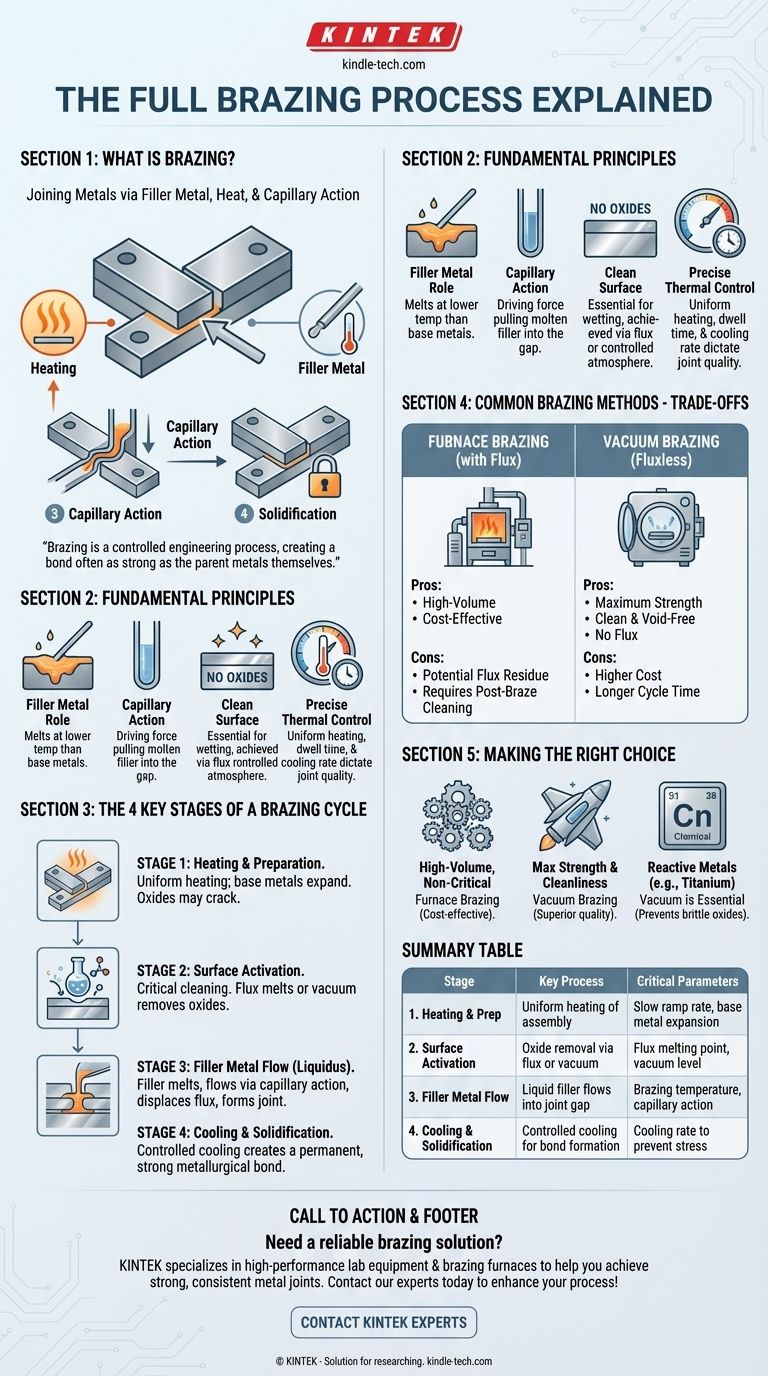
At its core, the brazing process is a method of joining metals by heating them and using a filler metal that melts at a lower temperature than the base metals. The molten filler is drawn into the tight gap between the parts through capillary action, and upon cooling, it solidifies to form a strong, permanent metallurgical bond. This entire sequence is performed under precise thermal control to ensure the integrity of the final joint.
Brazing is more than simply melting a filler material. It is a controlled engineering process that relies on the interplay between clean surfaces, precise temperature, and the physical force of capillary action to create a bond that is often as strong as the parent metals themselves.
The Fundamental Principles of Brazing
To fully understand the brazing process, you must grasp the core principles that govern its success. It is not a simple gluing operation but a complex interaction at the molecular level.
The Role of the Filler Metal
The entire process hinges on the filler metal, also called a braze alloy. It is specifically designed to have a melting point that is lower than the base metals being joined. This ensures the components being joined do not melt or deform during the process.
Capillary Action: The Driving Force
Brazing works because of a phenomenon called capillary action. A very small, uniform gap is designed between the two workpieces. When the filler metal melts and becomes liquid, it is automatically pulled into this gap, completely filling the joint regardless of gravity's orientation.
The Importance of a Clean Surface
For a strong bond to form, the molten filler metal must be able to "wet" the surfaces of the base metals. This is only possible if the surfaces are completely free of oxides and contaminants. Brazing processes achieve this in one of two ways: flux or a controlled atmosphere.
Precise Thermal Control
The final critical element is the control of heat. The assembly must be heated uniformly to the "brazing temperature"—above the filler's melting point but below the base metals'. The dwell time at this temperature and the subsequent cooling rate are essential parameters that dictate the final strength and microstructure of the joint.
The Four Key Stages of a Brazing Cycle
While specific parameters vary, nearly every brazing operation follows a consistent four-stage thermal cycle.
Stage 1: Heating and Preparation
The entire assembly is slowly and uniformly heated. As the temperature rises, the base metals expand. In processes using flux, this thermal expansion can cause the brittle oxide layer on the metal's surface to crack, providing an initial path for the flux to penetrate.
Stage 2: Surface Activation
This is the most critical cleaning phase.
- In flux-based brazing, the flux melts (e.g., at 565-572°C for aluminum) and becomes chemically active, aggressively dissolving and displacing surface oxides.
- In vacuum brazing, the low-pressure environment itself serves this purpose by deoxidizing the metals and vaporizing contaminants.
Stage 3: Filler Metal Flow (Liquidus)
As the temperature continues to rise to the specified brazing temperature (e.g., 577-600°C for some aluminum alloys), the filler metal melts. Driven by capillary action, the now-liquid filler flows into the clean, narrow gap between the workpieces, displacing the liquid flux and forming the joint.
Stage 4: Cooling and Solidification
The assembly is cooled in a controlled manner. The filler metal solidifies, creating a solid, permanent metallurgical bond between the two base metals. Proper cooling prevents thermal stress and ensures the desired properties of the final joint.
Understanding the Trade-offs: Common Brazing Methods
The "full process" also involves understanding how it is applied, as the method chosen has significant implications.
Furnace Brazing (with Flux or Atmosphere)
In furnace brazing, the entire assembly is placed inside a furnace and heated. This method is exceptionally efficient for high-volume production, as it can create thousands of joints simultaneously. When performed in open air, it requires the use of flux to protect the joint area from oxidation.
The primary trade-off is the potential for flux residue. This residue is often corrosive and must be thoroughly cleaned in a post-brazing operation, adding a step and cost to the process.
Vacuum Brazing (Fluxless)
Vacuum brazing is performed inside a high-vacuum chamber. The vacuum itself prevents oxidation, completely eliminating the need for flux. This results in exceptionally clean, strong, and void-free joints, making it the preferred method for critical, high-performance applications in aerospace, medical, and electronics.
The trade-off is cost and cycle time. Vacuum furnaces represent a significant capital investment, and the process of drawing a vacuum and running the thermal cycle is slower than atmospheric furnace brazing.
Making the Right Choice for Your Goal
Understanding the complete process allows you to select the right approach based on your project's specific requirements for cost, volume, and performance.
- If your primary focus is high-volume production for non-critical parts: Traditional furnace brazing with flux is often the most cost-effective method, but be sure to factor in post-braze cleaning requirements.
- If your primary focus is maximum joint strength and cleanliness: Vacuum brazing is the superior choice, delivering premium results by eliminating the risk of flux entrapment and contamination.
- If you are joining reactive metals like titanium or superalloys: A controlled atmosphere, especially a vacuum, is not optional—it is essential to prevent the formation of brittle oxides that would compromise the joint.
Mastering the brazing process means engineering the joint from the start by controlling these fundamental variables to achieve a reliable and robust connection.
Summary Table:
| Stage | Key Process | Critical Parameters |
|---|---|---|
| 1. Heating & Prep | Uniform heating of assembly | Slow ramp rate, base metal expansion |
| 2. Surface Activation | Oxide removal via flux or vacuum | Flux melting point, vacuum level |
| 3. Filler Metal Flow | Liquid filler flows into joint gap | Brazing temperature, capillary action |
| 4. Cooling & Solidification | Controlled cooling for bond formation | Cooling rate to prevent stress |
| Method Comparison | Furnace Brazing | Vacuum Brazing |
| High-volume, cost-effective | Maximum strength, flux-free, clean | |
| Requires post-braze flux cleaning | Higher cost, longer cycle time |
Need a reliable brazing solution for your laboratory or production line? KINTEK specializes in high-performance lab equipment, including brazing furnaces and consumables, to help you achieve strong, consistent metal joints. Whether you require high-volume furnace brazing or precision vacuum brazing for critical applications, our expertise ensures optimal results. Contact our experts today to discuss your specific brazing requirements and discover how KINTEK can enhance your joining process.
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Brazing Furnace
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- Why are vacuum furnaces or high-purity inert atmosphere furnaces required for joining refractory metals?
- What is the advantage of resistance furnace? Achieve Superior Control and Cleanliness
- What is the temperature of a brazed joint? Master the Key to Strong, Reliable Metal Joining
- What is the primary function of industrial ovens in lignocellulosic waste pretreatment? Maximize Energy Efficiency
- What is the purpose of the calcination process? A Guide to Purifying and Transforming Materials
- What is the necessity of using a vacuum oven for drying copper films? Ensure Oxidation-Free Material Preparation
- What are the main advantages of sintering? Achieve Complex Parts with Superior Material Properties
- How does a vacuum drying oven contribute to the preparation of cathodes for all-solid-state batteries? Pure Electrodes



















