High-temperature sintering of ceramics is a thermal treatment process used to convert a loosely packed ceramic powder into a dense, solid object. By applying heat at temperatures below the material's melting point, the individual powder particles are fused, significantly enhancing the material's mechanical strength, hardness, and thermal stability.
The core principle of sintering is not to melt the material, but to use thermal energy to drive atomic diffusion. This process eliminates the empty spaces between powder particles, creating strong chemical bonds and forming a solid, coherent mass.

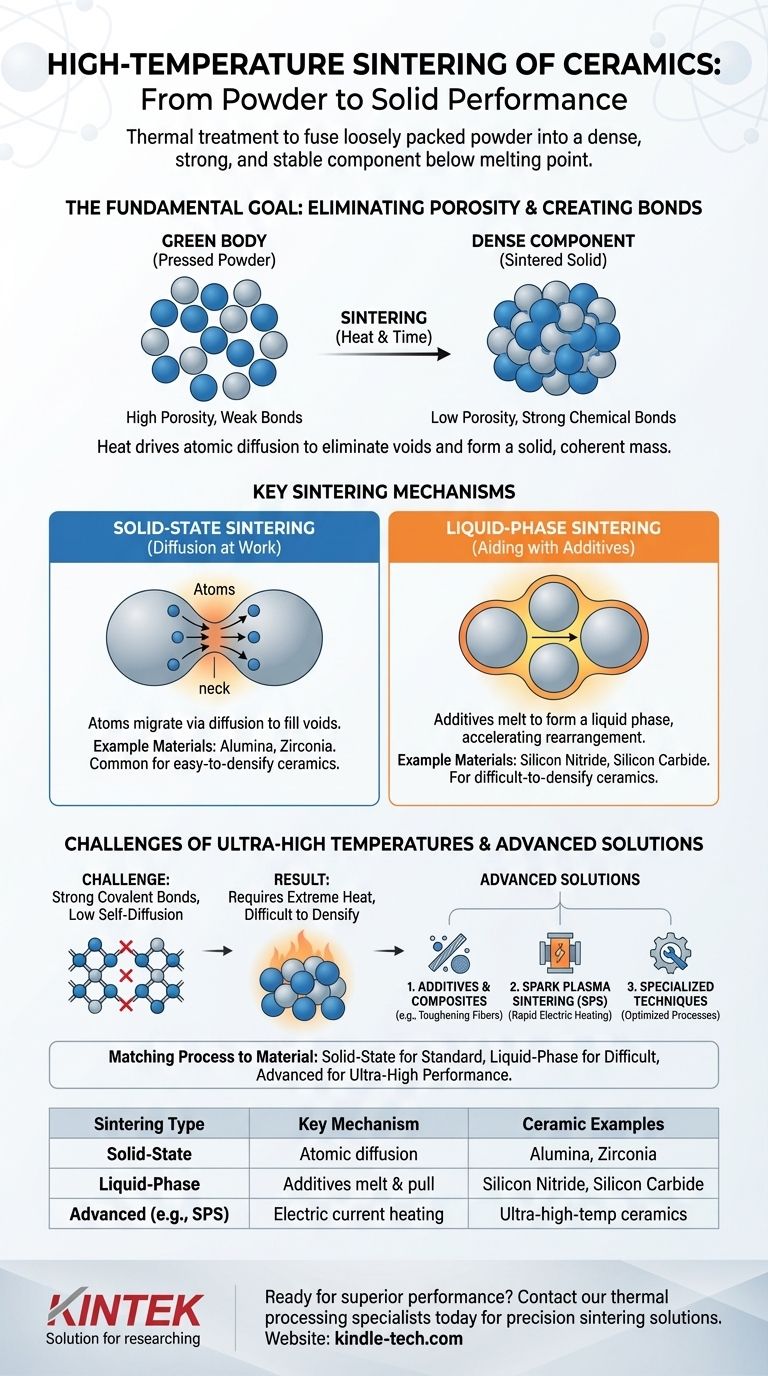
The Fundamental Goal: From Powder to Solid
Sintering is the critical manufacturing step that transforms a shaped part made of pressed powder—often called a "green body"—into a durable, high-performance ceramic component. The primary objective is to achieve maximum density.
Eliminating Porosity
The initial green body is highly porous, filled with air gaps between each particle. Sintering systematically reduces and eliminates this porosity, causing the component to shrink and become much denser.
Creating Strong Bonds
Heat provides the energy for atoms to move across the boundaries of adjacent particles. This atomic transport creates new, strong chemical bonds, effectively welding the particles together into a solid polycrystalline structure.
Key Sintering Mechanisms
The way atoms move to densify the material depends on the ceramic's intrinsic properties. The two primary mechanisms are solid-state and liquid-phase sintering.
Solid-State Sintering (Diffusion at Work)
For ceramics like alumina and zirconia, densification occurs entirely in the solid state. Atoms migrate from the bulk of the particles to the "necks" forming between them, gradually filling the voids through a process called diffusion.
This method relies entirely on the material's natural tendency for atoms to move at high temperatures.
Liquid-Phase Sintering (Aiding with Additives)
For ceramics that are notoriously difficult to densify, such as silicon nitride and silicon carbide, a different approach is used. Additives are mixed with the initial powder.
At sintering temperature, these additives melt to form a small amount of liquid. This liquid wets the ceramic particles and pulls them together through capillary forces, dramatically accelerating rearrangement and densification.
The Unique Challenges of Ultra-High Temperatures
Sintering certain advanced ceramics presents significant challenges due to their fundamental material properties, requiring extreme temperatures and specialized techniques.
Strong Covalent Bonds
Materials like silicon carbide feature exceptionally strong covalent bonds. These bonds lock atoms in place, making it incredibly difficult for them to diffuse, which is the very mechanism needed for solid-state sintering.
Low Self-Diffusion Rates
Because of these strong bonds, such materials have very low self-diffusion rates. This inherent resistance to atomic movement means that extremely high temperatures are needed to force densification, and even then, achieving full density can be difficult, sometimes resulting in lower fracture toughness.
Advanced Solutions
To overcome these issues, engineers use strategies like adding toughening fibers to create composite materials. They also employ advanced processes like Spark Plasma Sintering (SPS), which uses an electric current to rapidly heat the material, achieving high density at relatively lower temperatures and in much shorter times.
Matching the Process to the Material
The choice of sintering method is dictated by the properties of the ceramic and the performance requirements of the final component.
- If your material is relatively easy to densify (e.g., alumina): Standard solid-state sintering is the most direct and effective path to achieving a dense final product.
- If your material is highly resistant to densification (e.g., silicon carbide): Liquid-phase sintering is often necessary to facilitate particle rearrangement and achieve high density efficiently.
- If your goal is maximum performance from an ultra-high-temperature ceramic: Advanced techniques like composite reinforcement or Spark Plasma Sintering are required to overcome the material's inherent limitations.
Ultimately, sintering is the essential art of transforming loose powder into a precisely engineered, high-performance solid.
Summary Table:
| Sintering Type | Key Mechanism | Common Ceramic Examples |
|---|---|---|
| Solid-State Sintering | Atomic diffusion in solid state | Alumina, Zirconia |
| Liquid-Phase Sintering | Additives melt to form a liquid phase | Silicon Nitride, Silicon Carbide |
| Advanced (e.g., SPS) | Electric current for rapid heating | Ultra-high-temperature ceramics |
Ready to achieve superior material performance?
The precise thermal control required for successful ceramic sintering is critical. KINTEK specializes in high-performance lab furnaces and equipment designed for demanding processes like high-temperature sintering. Whether you are working with alumina, silicon carbide, or advanced composites, our solutions provide the uniform heating and temperature stability you need to transform your ceramic powders into dense, reliable components.
Let's discuss how our sintering expertise can enhance your R&D or production. Contact our thermal processing specialists today for a personalized consultation.
Visual Guide
Related Products
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- Vertical Laboratory Tube Furnace
- Laboratory High Pressure Vacuum Tube Furnace
People Also Ask
- What is the temperature of heat treatment? It Depends on Your Metal and Desired Properties
- What are the limitations of melting point determination? Understand Purity, Technique, and Material Behavior
- What is sputter ceramic film? A High-Tech Solution for Superior Heat Rejection & Clarity
- What is sputter coating SEM sample preparation? Eliminate Charging for Crisp, Stable Images
- How does heat treating affect the strength of a metal? A Guide to Tailoring Metal Properties
- What is the double sintering method? Achieve Maximum Density with Controlled Microstructure
- What are the two important principles of heat treatment? Achieve Optimal Material Properties for Your Lab
- Which method is best for removing a solid from a liquid? A Guide to Filtration, Decantation, Evaporation & Centrifugation



















