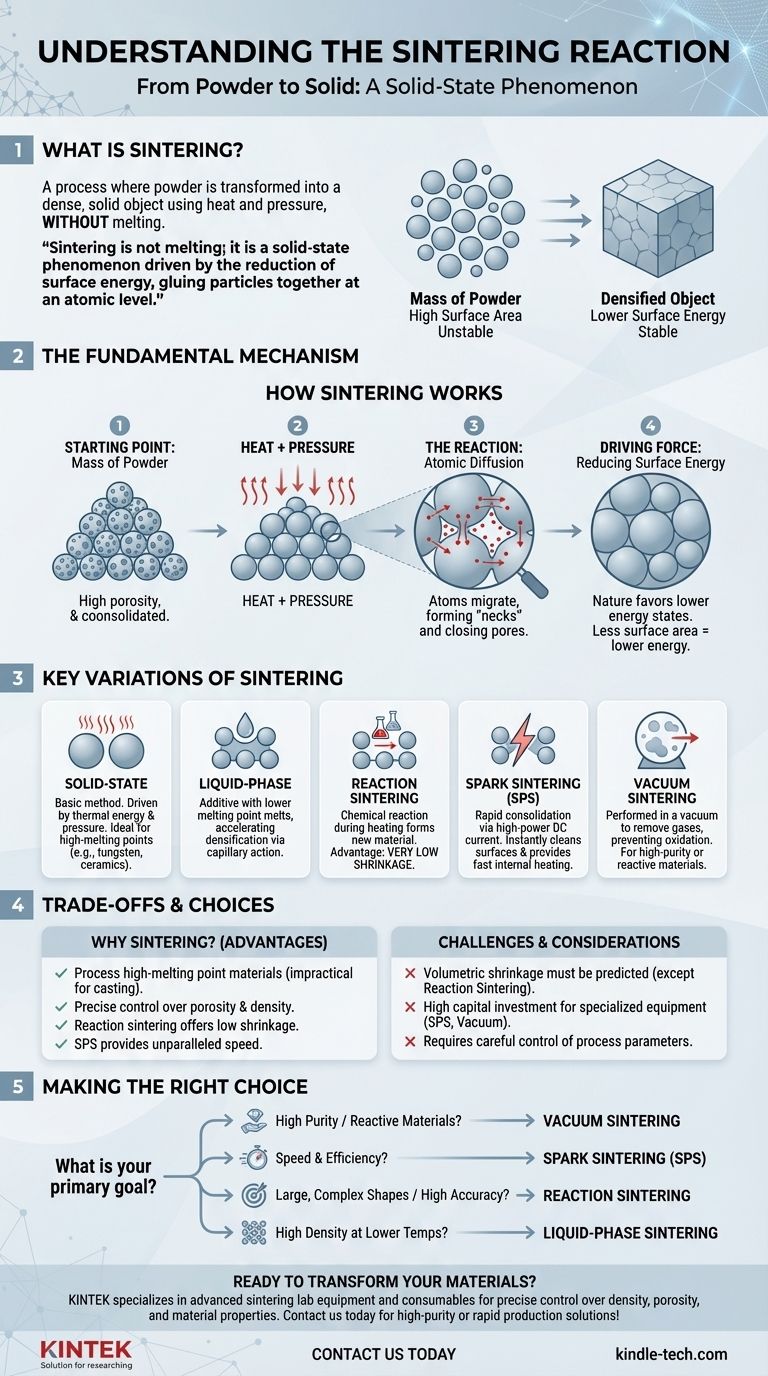
At its core, a sintering reaction is the process where a mass of powder is transformed into a solid, dense object using heat and pressure. Crucially, this is achieved without melting the material into a liquid. The "reaction" is the atomic diffusion that occurs across the boundaries of individual particles, causing them to fuse together into a single, cohesive piece.
Sintering is not a melting process; it is a solid-state phenomenon driven by the reduction of surface energy. It "glues" particles together at an atomic level, allowing the creation of robust components from powders, which is especially vital for materials with extremely high melting points.

The Fundamental Mechanism: How Sintering Works
To understand sintering, you must visualize it as a microscopic construction process where individual particles are the building blocks. The goal is to eliminate the empty spaces between them.
The Starting Point: A Mass of Powder
The process begins with a collection of individual powder particles. This loose or lightly compacted material has a very high total surface area and significant internal porosity (empty space). This high surface area represents a state of high surface energy, which is inherently unstable.
The Role of Heat and Pressure
The powder is subjected to high temperatures, which gives the atoms within the particles enough energy to become mobile. This temperature is kept below the material's melting point. Simultaneously, pressure may be applied to force the particles into close contact, reducing the distance the atoms need to travel.
The "Reaction": Atomic Diffusion
With atoms energized and particles in contact, diffusion begins. Atoms migrate from one particle to another across their shared boundaries. This movement of matter starts to form small physical bridges, or "necks," between adjacent particles.
The Driving Force: Reducing Surface Energy
The fundamental reason sintering occurs is that nature favors lower energy states. A single, solid object has far less surface area than the countless individual powder particles it was made from. By forming necks and eliminating internal pores, the system dramatically reduces its total surface energy, providing the thermodynamic driving force for the entire process.
Key Variations of the Sintering Process
While the core principle remains the same, several specialized techniques have been developed to optimize the process for different materials and outcomes.
Solid-State Sintering
This is the most basic form of sintering, relying purely on thermal energy and external pressure to drive atomic diffusion between solid particles. It is widely used for ceramics and metals like tungsten, which have melting points too high for conventional casting.
Liquid-Phase Sintering
In this method, a small amount of an additive with a lower melting point is mixed with the primary powder. At the sintering temperature, this additive melts, creating a liquid that wets the solid particles. This liquid phase accelerates densification through capillary action, which pulls particles together, and by acting as a fast transport path for dissolving and re-precipitating material into the pores.
Reaction Sintering
This technique involves a chemical reaction during the heating process. For example, a porous silicon shape can be infiltrated with carbon and heated, causing a reaction that forms new silicon carbide (SiC) in the pores. A major advantage is very low shrinkage, making it ideal for producing large or complex-shaped parts with high precision.
Spark Sintering (SPS)
Also known as Spark Plasma Sintering, this is a rapid consolidation technique. A high-power DC electrical current is passed directly through the powder while it is under pressure. The current instantly burns away surface contaminants on the particles and provides extremely fast, internal heating, allowing parts to be fully sintered in a matter of seconds or minutes.
Vacuum Sintering
This process is performed inside a vacuum furnace. The primary purpose of the vacuum is to remove atmospheric gases like oxygen and nitrogen. This prevents unwanted chemical reactions, such as oxidation, which is critical when working with reactive materials or when the final product requires very high purity.
Understanding the Trade-offs
Sintering is a powerful manufacturing tool, but its use is dictated by a clear set of advantages and limitations.
Why Not Just Melt and Cast?
The primary advantage of sintering is its ability to process materials with extremely high melting points, such as tungsten, molybdenum, and many advanced ceramics. These materials are impractical or impossible to shape using traditional melting and casting methods.
Control Over Porosity and Density
Sintering offers precise control over the final part's density. The process can be stopped early to create parts with controlled porosity for applications like filters, or it can be run to completion to achieve nearly full theoretical density for structural components.
The Challenge of Shrinkage
As the pores between particles are eliminated, the overall component shrinks. This volumetric shrinkage is a critical design consideration and must be accurately predicted and compensated for in the initial powder compact's design. Reaction sintering is a notable exception with minimal shrinkage.
Cost and Process Complexity
While some methods are cost-effective, specialized equipment for processes like Spark Sintering or Vacuum Sintering represents a significant capital investment. The process requires careful control over the temperature profile, pressure, time, and atmosphere to achieve consistent and reliable results.
Making the Right Choice for Your Goal
Selecting the correct sintering method depends entirely on the material you are using and the desired properties of the final component.
- If your primary focus is working with high-purity or reactive materials: Vacuum Sintering is necessary to prevent contamination and oxidation during the process.
- If your primary focus is speed and manufacturing efficiency: Spark Sintering (SPS) offers unparalleled speed, consolidating powders into dense parts in seconds.
- If your primary focus is creating large, complex shapes with high dimensional accuracy: Reaction Sintering is the superior choice due to its inherently low shrinkage.
- If your primary focus is achieving high density at lower temperatures: Liquid-Phase Sintering can accelerate the process and enable full densification more easily than solid-state methods.
Understanding the specific mechanism of sintering empowers you to select the precise manufacturing process required to transform simple powders into high-performance components.
Summary Table:
| Sintering Method | Key Mechanism | Primary Use Case |
|---|---|---|
| Solid-State | Atomic diffusion driven by heat/pressure | High-melting point ceramics & metals (e.g., tungsten) |
| Liquid-Phase | Liquid additive accelerates particle bonding | Achieving high density at lower temperatures |
| Reaction | Chemical reaction forms new material in pores | Large/complex shapes with minimal shrinkage |
| Spark Plasma (SPS) | Rapid internal heating via electric current | Fast consolidation for manufacturing efficiency |
| Vacuum | Prevents oxidation in a gas-free environment | High-purity or reactive materials |
Ready to transform your powder materials into high-performance components? KINTEK specializes in lab equipment and consumables, providing advanced sintering solutions tailored to your laboratory's needs. Whether you require vacuum furnaces for high-purity results or Spark Plasma Sintering systems for rapid production, our expertise ensures precise control over density, porosity, and material properties. Contact us today to discuss how our sintering technology can enhance your research and manufacturing outcomes!
Visual Guide
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Brazing Furnace
People Also Ask
- What is the sintering time? A Critical Process Variable for Material Density and Strength
- What are the defects in sintered parts? Avoid Warping, Cracking, and Porosity Issues
- Does sintering use diffusion? The Atomic Mechanism for Building Stronger Materials
- Why is high-temperature vacuum heat treatment critical for Cr-Ni steel? Optimize Strength & Surface Integrity
- Why is environmental control within a vacuum furnace important for diffusion bonding? Master Titanium Alloy Laminates



















