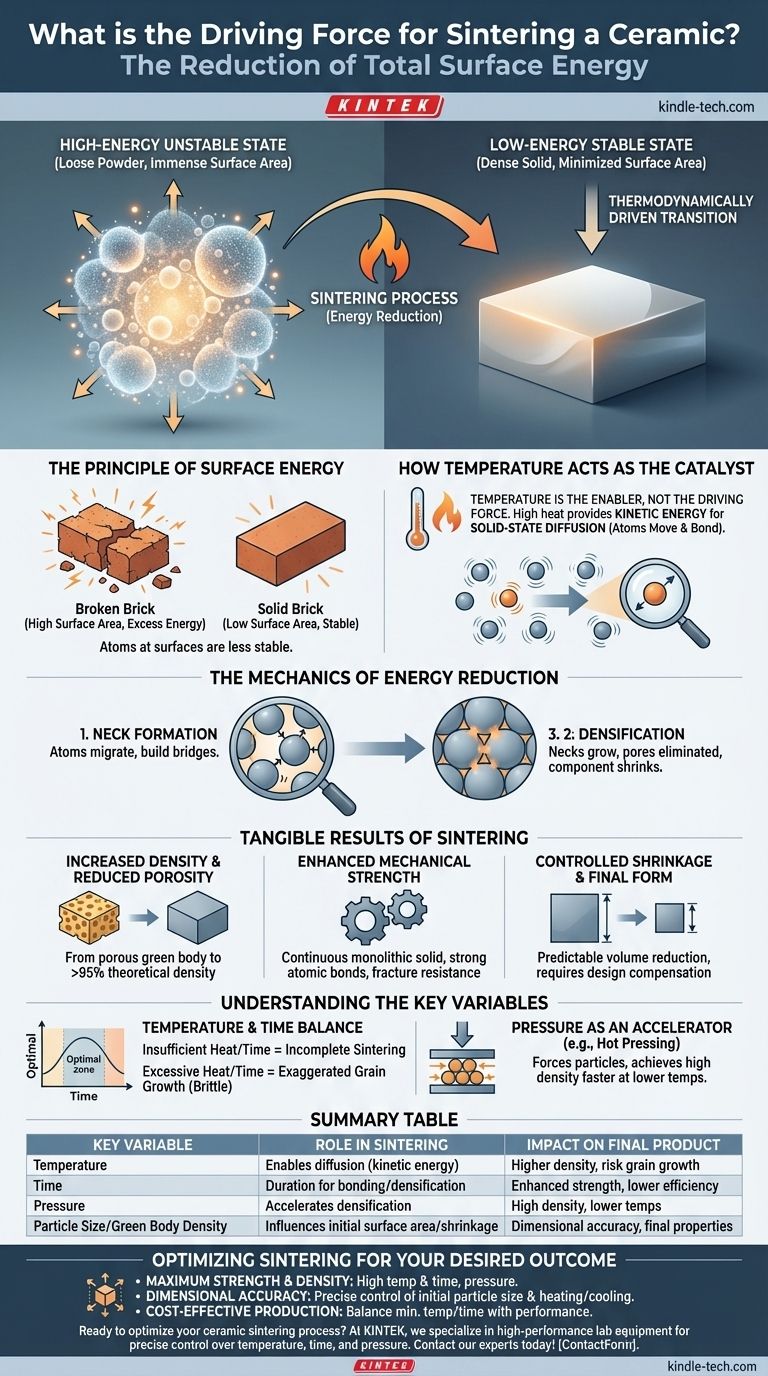
At its core, the driving force for sintering a ceramic is the reduction of total surface energy. When a collection of fine ceramic particles is heated, the system naturally seeks a lower, more stable energy state. It achieves this by fusing the particles together, which dramatically decreases their collective surface area and eliminates the empty space between them.
A loose powder possesses an immense amount of surface area, which corresponds to a high-energy, unstable state. Sintering is the thermodynamically driven process of transforming this high-energy powder into a low-energy, dense solid by using high temperature to enable atoms to move and bond.

The Principle of Surface Energy
To understand sintering, you must first understand the fundamental instability of a powder. The process is a natural consequence of the material attempting to achieve a more stable configuration.
What is Surface Energy?
Imagine a single brick versus that same brick ground into a fine powder. While the mass is identical, the powder has a vastly larger total surface area. Each new surface created during grinding requires energy, and the atoms at these surfaces are less stable than those in the interior of a solid.
This excess energy stored on the surfaces of the powder particles is called surface energy. A system with high surface energy is inherently unstable and will, if given a path, transition to a lower energy state.
How Temperature Acts as the Catalyst
Temperature itself is not the driving force; it is the enabler. High heat provides the atoms within the ceramic particles with enough kinetic energy to move, a process known as solid-state diffusion.
Without sufficient heat, the atoms are locked in place, and the powder remains a powder. Temperature unlocks their ability to migrate and rearrange the structure to reduce the total surface energy.
The Mechanics of Energy Reduction
Under high heat, two key things happen at the atomic level:
- Neck Formation: Atoms migrate from the surface of the particles to the points of contact between them. This builds small bridges, or "necks," between adjacent particles.
- Densification: As these necks grow, they pull the centers of the particles closer together. This systematically eliminates the voids (or pores) between the particles, causing the entire component to shrink and become denser.
The Tangible Results of Sintering
This fundamental drive to reduce energy results in profound changes to the material's physical and mechanical properties.
Increased Density and Reduced Porosity
The most direct outcome is the transformation of a porous "green body" (the compacted but unfired part) into a dense solid. Porosity is drastically reduced, often achieving a final material that is over 95% of its theoretical maximum density. This is what turns a chalky, fragile object into a hard, solid component.
Enhanced Mechanical Strength
A powder has virtually no mechanical strength. The sintering process creates a continuous, monolithic solid with strong atomic bonds where particle boundaries once were.
Furthermore, the pores that are eliminated during sintering act as microscopic stress concentrators. Removing them dramatically increases the material's resistance to fracture, resulting in the high strength characteristic of engineering ceramics.
Controlled Shrinkage and Final Form
Because sintering eliminates the space between particles, the overall volume of the component is reduced. This shrinkage is a critical and predictable part of the process.
Engineers must design the initial green body to be proportionally larger than the desired final part to account for this volumetric reduction.
Understanding the Key Variables
Controlling the sintering process is essential for achieving the desired final properties. The primary variables are temperature, time, and sometimes pressure.
The Impact of Temperature and Time
There is a delicate balance between temperature and time. Insufficient heat or a cycle that is too short will result in incomplete sintering, leaving behind residual porosity that compromises strength and density.
Conversely, excessively high temperatures or holding the part at temperature for too long can cause exaggerated grain growth. While the part is dense, these overly large grains can create internal stresses and actually reduce the material's toughness, making it more brittle.
Pressure as an Accelerator
Some advanced sintering processes, like hot pressing, apply external pressure in addition to high temperature. This pressure physically forces the particles together, accelerating densification.
This technique can achieve very high densities at lower temperatures or in shorter times than conventional sintering, and it is often used for high-performance or difficult-to-sinter materials.
Optimizing Sintering for Your Desired Outcome
The "ideal" sintering cycle depends entirely on the goal for the final product. By manipulating the core variables, you can tailor the outcome to your specific application.
- If your primary focus is maximum strength and density: Optimize for high temperatures and sufficient time to eliminate nearly all porosity, potentially incorporating pressure for critical applications.
- If your primary focus is dimensional accuracy: You must precisely control the initial particle size distribution, green body density, and the heating/cooling rates to manage shrinkage predictably.
- If your primary focus is cost-effective production: The goal is to find the minimum temperature and time required to achieve acceptable properties, balancing energy costs against the required performance of the final part.
Ultimately, understanding that sintering is a process of energy reduction empowers you to manipulate temperature and time to engineer ceramic materials with precisely the properties you require.
Summary Table:
| Key Variable | Role in the Sintering Process | Impact on Final Product |
|---|---|---|
| Temperature | Enables atomic diffusion by providing kinetic energy | Higher temps increase density but risk grain growth |
| Time | Determines duration for particle bonding and densification | Longer times enhance strength but can reduce efficiency |
| Pressure (e.g., Hot Pressing) | Accelerates densification by forcing particles together | Achieves high density at lower temperatures |
| Particle Size/Green Body Density | Influences initial surface area and shrinkage control | Critical for dimensional accuracy and final properties |
Ready to optimize your ceramic sintering process for maximum strength, density, or cost-efficiency? At KINTEK, we specialize in high-performance lab equipment, including sintering furnaces and consumables, to help you achieve precise control over temperature, time, and pressure. Whether you're developing advanced ceramics or scaling production, our solutions ensure reliable, repeatable results. Contact our experts today to discuss how we can support your laboratory's sintering needs!
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the effect of calcination? Unlock Material Transformation for Industrial Processes
- What is the material used in muffle furnace? Discover the Heat-Resistant Layers Inside
- What is the effect of calcination temperature on the properties of nanoparticles? Master the Trade-Off for Optimal Performance
- What is the design and construction of a muffle furnace? A Guide to Its Isolated Heating Chamber
- What is a muffle furnace used for in pharma? Ensuring Purity and Regulatory Compliance



















