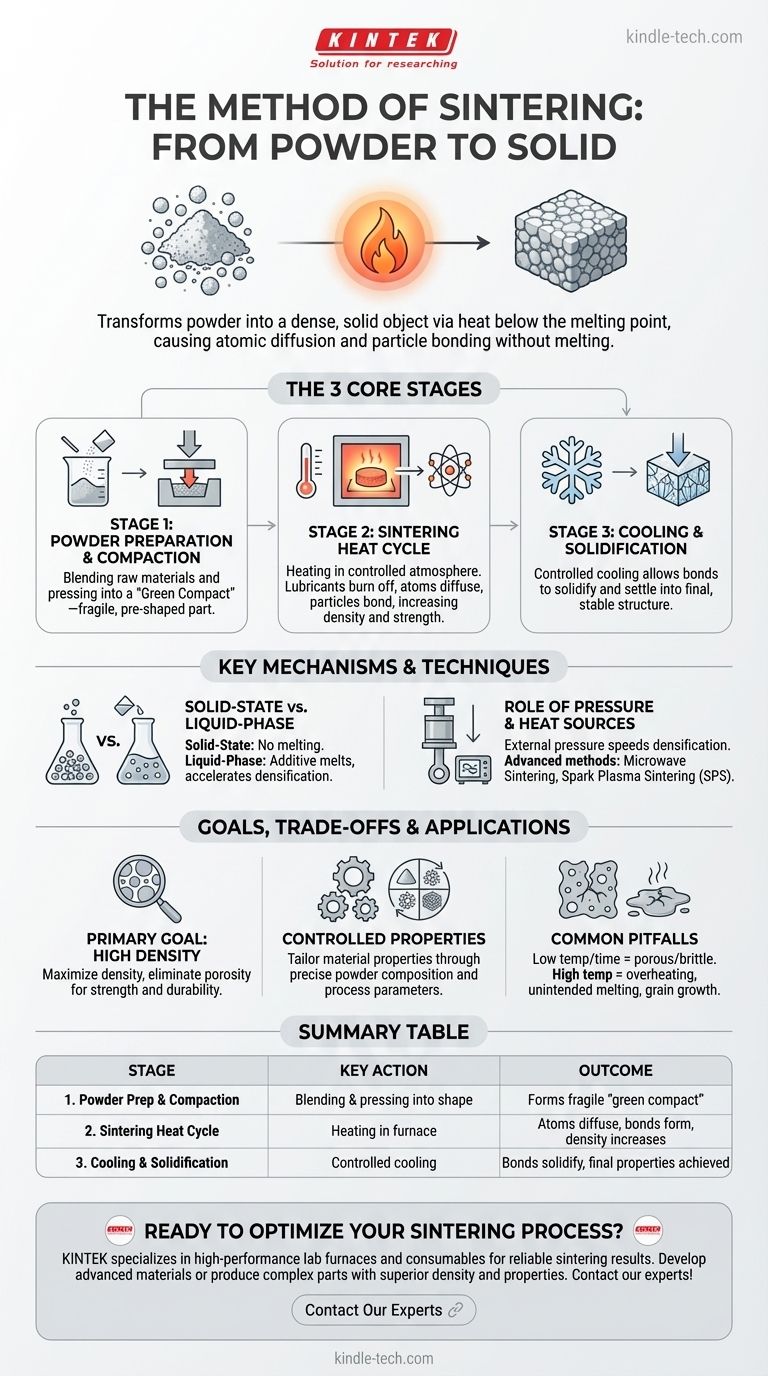
In short, the method of sintering transforms a powder into a solid, dense object by applying heat at a temperature below the material's melting point. This process causes the individual particles of the powder to bond together, eliminating the empty spaces between them and creating a unified mass with desired mechanical properties. It is a foundational technique used in fields ranging from metallurgy to the production of ceramics.
The critical distinction to understand is that sintering is not about melting. It is a solid-state process where atomic diffusion—driven by heat—causes powder particles to fuse, creating a strong, dense component without ever turning the bulk material into a liquid.

The Core Stages of the Sintering Process
Sintering is a multi-stage process. While the specifics vary, the fundamental progression from powder to solid part remains consistent and can be broken down into three primary stages.
Stage 1: Powder Preparation and Compaction
Before any heating occurs, the raw material must be prepared. This involves blending the primary powdered material (like a metal or ceramic) with any necessary alloying elements or additives.
This powder mixture is then pressed into the desired shape using a die, mold, or other tooling. This initial pressing, often done at room temperature, creates what is known as a "green compact"—a fragile part that holds its shape but has not yet developed its final strength.
Stage 2: The Sintering Heat Cycle
The green compact is placed into a furnace and heated in a controlled atmosphere. The temperature is raised to a specific point that is high enough to energize the atoms but remains safely below the material's melting point.
During this stage, several crucial events happen. Any lubricants or binding agents from the compaction stage are burned off. Most importantly, atoms begin to diffuse across the boundaries of the individual particles, creating strong metallurgical bonds where they touch. This process of atomic bonding closes the gaps and pores between particles, increasing the object's density and strength.
Stage 3: Cooling and Solidification
After holding the part at the sintering temperature for a designated period, it is cooled in a controlled manner. This final stage allows the newly formed bonds to solidify and the material to settle into its final, stable crystalline structure. The resulting object is a single, unified mass with its intended mechanical properties.
Key Sintering Mechanisms and Techniques
The general method of sintering serves as a foundation for numerous specialized techniques. The choice of technique depends on the material being used, the desired final properties, and production efficiency goals.
Solid-State vs. Liquid-Phase Sintering
The most fundamental distinction is whether any part of the material melts. In solid-state sintering, the entire process occurs without any melting. In liquid-phase sintering (LPS), an additive with a lower melting point is included in the powder mix. During heating, this additive melts and flows into the pores between the solid particles, accelerating the densification process.
The Role of Pressure and Heat Sources
While conventional sintering relies primarily on heat in a furnace, advanced methods manipulate other variables. Applying external pressure during the heating cycle can significantly speed up densification and achieve higher final densities. Likewise, the heat source itself can be changed.
Common Sintering Methods
Beyond conventional furnace heating, several modern techniques exist:
- Microwave Sintering: Uses microwaves to heat the material internally and uniformly, often resulting in faster processing times.
- Spark Plasma Sintering (SPS): Passes a pulsed electrical current directly through the powder while applying pressure. This generates rapid, intense heat precisely at the particle contact points, enabling extremely fast sintering.
Understanding the Trade-offs and Goals
Sintering is not used arbitrarily; it is chosen to solve specific engineering challenges, but it comes with its own considerations.
The Primary Goal: Achieving High Density
The core objective of sintering is to reduce or eliminate porosity (the empty space between particles). A dense, non-porous part is typically stronger and more durable. The success of a sintering process is often measured by the final density achieved relative to the theoretical maximum density of the material.
Control Over Material Properties
Sintering provides exceptional control over the final properties of a component. By carefully selecting the initial powder composition, compaction pressure, temperature, and time, engineers can create materials with tailored characteristics that would be difficult or impossible to achieve through traditional casting or machining.
Common Pitfalls to Avoid
The main risk in sintering is failing to achieve adequate densification. If the temperature is too low or the time too short, the bonds between particles will be weak, leaving the final part porous and brittle. Conversely, if the temperature is too high, unintended melting or grain growth can occur, degrading the material's properties.
Applying Sintering to Your Goal
The right approach to sintering depends entirely on the intended outcome.
- If your primary focus is producing complex metal parts at scale: Conventional powder metallurgy, which relies on compaction and furnace sintering, is the established, cost-effective method.
- If your primary focus is developing advanced materials with unique properties: Explore modern techniques like Spark Plasma Sintering (SPS) for finer control, higher densities, and faster processing.
- If your primary focus is creating durable ceramic components: Recognize that the core principle of fusing particles with heat is the key to transforming brittle powders into hard, resilient final products.
Ultimately, understanding the method of sintering is about controlling heat, time, and pressure to build robust components from the particle level up.
Summary Table:
| Stage | Key Action | Outcome |
|---|---|---|
| 1. Powder Preparation & Compaction | Blending and pressing powder into a shape | Forms a fragile 'green compact' |
| 2. Sintering Heat Cycle | Heating in a controlled furnace atmosphere | Atoms diffuse, creating bonds and increasing density |
| 3. Cooling & Solidification | Controlled cooling of the part | Bonds solidify, final properties are achieved |
Ready to optimize your sintering process with precision equipment? KINTEK specializes in high-performance lab furnaces and consumables designed for reliable, consistent sintering results. Whether you are developing advanced materials or producing complex metal parts, our solutions help you achieve superior density and material properties. Contact our experts today to discuss your specific laboratory needs!
Visual Guide
Related Products
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Spark Plasma Sintering Furnace SPS Furnace
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- Why is a high vacuum environment necessary in sintering equipment for TiAl alloys? Ensure High-Purity Metal Bonding
- Why must green bodies produced via binder jetting undergo treatment in a vacuum sintering furnace?
- What is the standard thickness of plating? Optimize Durability, Corrosion & Cost
- How does precise temperature control affect FeCoCrNiMnTiC high-entropy alloys? Master Microstructural Evolution
- Why is a high vacuum required for sintering Ti-43Al-4Nb-1Mo-0.1B? Ensure Purity & Fracture Toughness



















