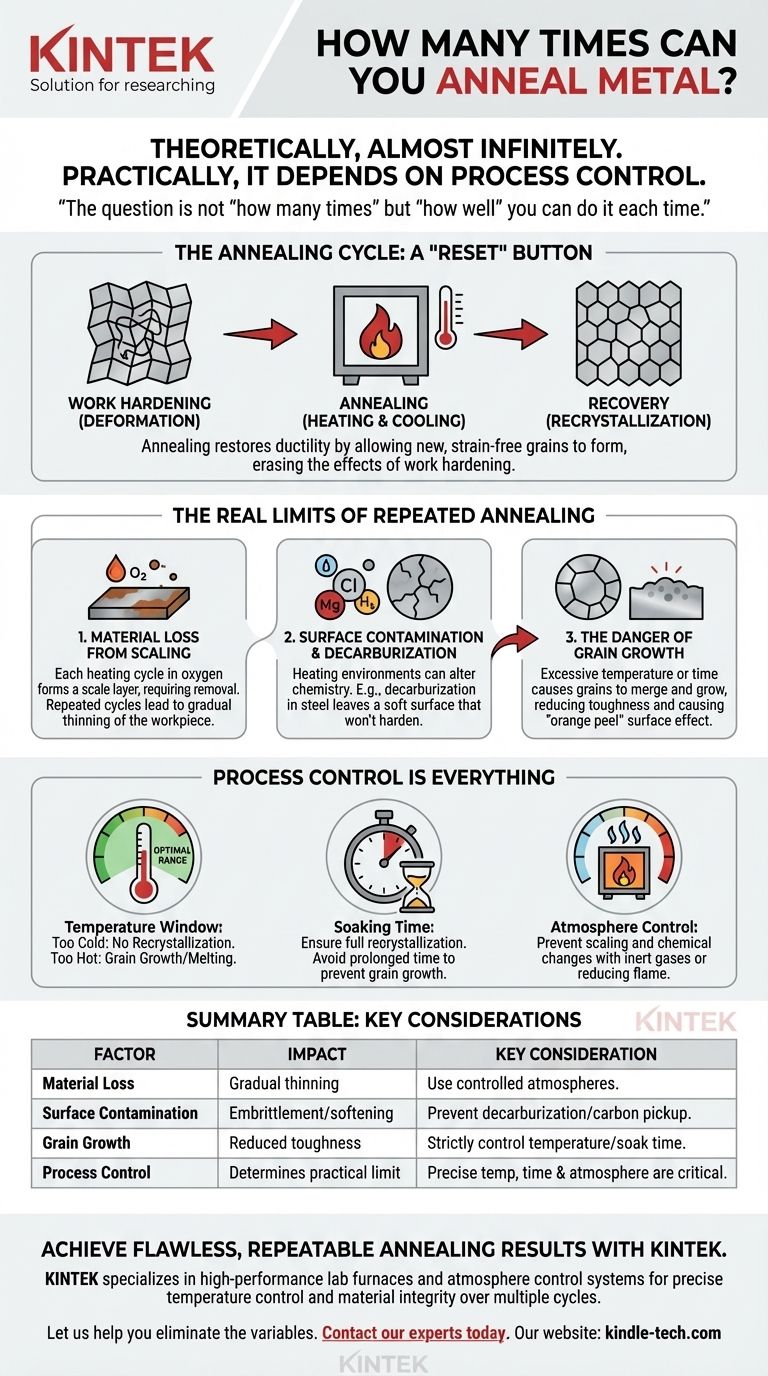
In principle, a pure metal can be annealed an almost infinite number of times. The process of annealing is a restorative one, designed to reset the metal's internal crystal structure after it has been work-hardened. However, the practical limit is not determined by the act of annealing itself, but by the precision and control of each heating and cooling cycle.
The question is not "how many times" you can anneal, but "how well" you can do it each time. The true limitations on repeated annealing are practical, not theoretical, and are caused by material loss, contamination, and grain growth from imperfect process control.
Why We Anneal: The Cycle of Work Hardening and Recovery
Understanding Work Hardening
When you bend, hammer, or draw metal, you are deforming its internal crystalline structure. This process, known as work hardening or strain hardening, makes the metal stronger and more brittle, resisting further shaping.
Internally, this happens because microscopic defects called dislocations become tangled, preventing crystal planes from slipping past one another easily.
The Role of Annealing: A "Reset" Button
Annealing is the controlled process of heating a metal to a specific temperature and then cooling it. This thermal energy allows the internal structure to repair itself, relieving stress and restoring the metal's ductility.
Essentially, annealing acts as a "reset" button, making the metal soft and workable again.
The Mechanism: Recrystallization
The magic of annealing happens through a process called recrystallization. At the target temperature, new, strain-free grains begin to form within the deformed structure.
These new grains consume the old, tangled ones, effectively erasing the effects of work hardening and returning the metal to its softest state.
The Real Limits of Repeated Annealing
While the annealing process itself is restorative, the practical execution introduces variables that can degrade the metal over many cycles.
Problem 1: Material Loss from Scaling
Each time a metal is heated in the presence of oxygen, its surface oxidizes, forming a layer of scale. This is especially true for copper, brass, and non-stainless steels.
This scale must be cleaned off, typically with an acid pickle or abrasion. Each cycle of heating and cleaning removes a small amount of material, which can become significant over dozens of cycles, thinning the workpiece.
Problem 2: Surface Contamination and Decarburization
The heating environment can alter the metal's chemistry. For example, a fuel-rich flame can introduce carbon into the surface of copper, causing embrittlement.
Conversely, for carbon steels, heating in an oxygen-rich environment can cause decarburization, where carbon is burned out of the surface. This leaves a soft iron layer that will not harden properly, compromising the finished part's integrity.
Problem 3: The Danger of Grain Growth
If a metal is heated above its recrystallization temperature or held at temperature for too long, the newly formed grains will start to merge and grow larger.
Excessive grain growth is detrimental. It can reduce the metal's toughness and strength. When bent, a metal with very large grains may exhibit a rough, bumpy surface texture known as an "orange peel" effect. This damage is generally irreversible.
Understanding the Trade-offs: Process Control is Everything
Your ability to anneal a piece of metal repeatedly without damaging it comes down to mastering three variables. Failure in any of these areas is what truly limits the lifespan of your workpiece.
The Temperature Window: Too Hot vs. Too Cold
Every alloy has a specific annealing temperature range.
- Too cold, and recrystallization will not occur, meaning the metal remains work-hardened.
- Too hot, and you risk severe grain growth or even melting the metal.
Using temperature-indicating crayons or a calibrated furnace is crucial for repeatable, non-damaging results.
The Time Factor: Soaking and Cooling
The metal must be held at the annealing temperature—a process called "soaking"—long enough for the entire cross-section to fully recrystallize. Thicker pieces require longer soak times.
However, once full recrystallization occurs, any additional time at temperature only contributes to undesirable grain growth. The cooling rate is also critical for some alloys and can affect the final properties.
The Environmental Factor: Atmosphere Control
Controlling the atmosphere during heating prevents scaling and chemical changes. For jewelers, this means using a neutral or slightly reducing flame.
In industrial settings, this is achieved by annealing inside furnaces filled with inert gases (like argon or nitrogen) to protect the metal's surface.
How to Apply This to Your Work
Your strategy for managing repeated annealing cycles depends on your material and goal.
- If your primary focus is jewelry or coppersmithing: Prioritize clean heating practices and accurate temperature control to minimize material loss from scaling and prevent overheating that causes grain growth.
- If your primary focus is blacksmithing with carbon steel: Pay close attention to your forge atmosphere and heating times to prevent decarburization, which will ruin the steel's ability to be hardened.
- If your primary focus is industrial forming of alloys: Implement precise, calibrated furnace controls for temperature, time, and atmosphere to ensure consistent, repeatable results without material degradation.
By mastering the annealing process, you gain control over the metal's fundamental properties, enabling you to shape it to your will.

Summary Table:
| Factor | Impact on Repeated Annealing | Key Consideration |
|---|---|---|
| Material Loss (Scaling) | Gradual thinning of workpiece | Use controlled atmospheres to minimize oxidation. |
| Surface Contamination | Embrittlement or softening | Prevent decarburization in steel; avoid carbon pickup in copper. |
| Grain Growth | Reduced toughness, 'orange peel' surface | Strictly control temperature and soak time. |
| Process Control | Determines practical limit | Precise temperature, time, and atmosphere are critical. |
Achieve flawless, repeatable annealing results with KINTEK.
Whether you're in jewelry making, blacksmithing, or industrial metal forming, precise temperature control and a protective atmosphere are non-negotiable for maintaining material integrity over multiple cycles. KINTEK specializes in high-performance lab furnaces and atmosphere control systems that deliver the accuracy and reliability your work demands.
Let us help you eliminate the variables that limit your process. Contact our experts today to find the perfect annealing solution for your laboratory or workshop.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What are muffle furnaces used for? Achieve Precise, Contaminant-Free High-Temperature Processing
- What is the temperature range of a laboratory muffle furnace? Find the Right Model for Your Application
- How does tempering reduce hardness? Achieve the Perfect Balance of Toughness and Durability
- What is the melting temperature of ceramics? Understanding High-Temperature Material Performance
- What is the muffle furnace used for ash content? Achieve Accurate Gravimetric Analysis



















