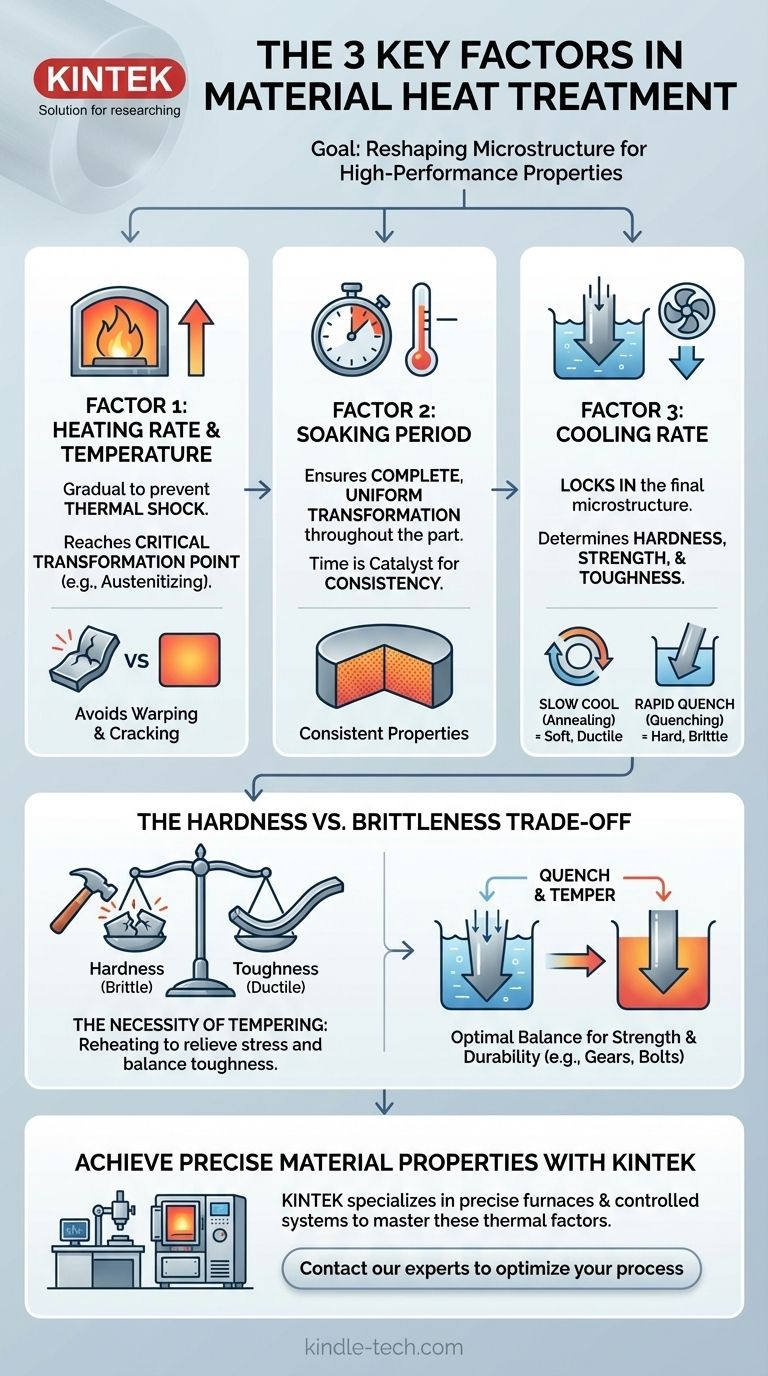
The three most important factors in any material heat treatment process are the heating rate and temperature, the soaking time at that temperature, and the subsequent cooling rate. These three variables are not merely sequential stages; they are the fundamental levers used to intentionally alter a metal's internal microstructure. Mastering control over them is how we transform a standard metal into a high-performance material with specific properties like hardness, toughness, or ductility.
The core principle of heat treatment is not just about changing a metal's temperature, but about precisely controlling the rate and duration of thermal changes to intentionally manipulate its microscopic crystal structure, thereby dictating its final mechanical properties.

The Goal: Reshaping a Metal's Internal Structure
Understanding Microstructure
At its core, heat treatment is the science of manipulating a metal's microstructure. This refers to the size, shape, and arrangement of the crystalline grains within the material.
The properties we observe on a macro level—such as hardness, strength, and brittleness—are a direct result of this internal architecture. Heat treatment provides the energy needed to dissolve old structures and form new ones.
Factor 1: The Heating Cycle
Why Gradual Heating is Critical
The first factor is the rate at which a material is heated to its target temperature. A slow, uniform heating process is essential to prevent thermal shock.
If a component is heated too quickly, the exterior will expand much faster than the cooler interior. This differential expansion creates immense internal stress, which can lead to warping, distortion, or even cracking before the real treatment begins.
Reaching the Transformation Temperature
The goal of heating is to bring the metal above a critical transformation temperature. For steel, this is known as the austenitizing temperature.
Above this point, the metal's default crystal structure (like ferrite and pearlite at room temperature) dissolves into a new, uniform solid-solution structure (austenite). This new structure is the necessary starting point for achieving desired properties upon cooling.
Factor 2: The Soaking Period
Temperature as the Primary Driver
Once the material reaches the target temperature, it is "soaked" or held at that temperature for a specific period. The exact temperature is paramount.
A slightly different soaking temperature can result in a completely different balance of properties. It dictates the extent to which elements dissolve and the potential for grain growth, which influences toughness.
Time as the Catalyst for Uniformity
Soaking time ensures that the transformation is complete and uniform throughout the entire cross-section of the part.
A thick component requires a longer soaking time than a thin one to ensure the core reaches the same temperature and completes its microstructural change as the surface. Insufficient soaking leads to inconsistent properties and unreliable performance.
Factor 3: The Cooling Rate
Locking in the Final Structure
The rate of cooling is arguably the most decisive factor in determining the final mechanical properties of the metal. This step "locks in" a specific microstructure by controlling how the atoms rearrange as they lose energy.
Different cooling rates produce vastly different outcomes from the exact same starting structure.
From Slow Cools to Rapid Quenches
A slow cool, such as letting the part cool in the furnace (annealing), allows the crystal structure to reform in a soft, low-stress, and highly ductile state.
A rapid cool, known as quenching (by plunging the part into water, oil, or polymer), is a violent process. It traps the atoms in a highly stressed, distorted crystal structure (like martensite in steel), which is extremely hard and brittle. The speed of the quench directly correlates to the level of hardness achieved.
Understanding the Trade-offs
Hardness vs. Brittleness
The most fundamental trade-off in heat treatment is between hardness and toughness. Processes that produce extreme hardness, like a rapid water quench, almost always result in high brittleness.
A harder material is more resistant to wear and deformation, but it is also more likely to fracture suddenly under impact or stress.
The Necessity of Tempering
Because a fully hardened, as-quenched part is often too brittle for practical use, a secondary heat treatment called tempering is required.
Tempering involves reheating the hardened part to a much lower temperature. This process relieves internal stresses and sacrifices some hardness to regain a crucial amount of toughness, creating a more durable and reliable final component.
Making the Right Choice for Your Goal
The ideal combination of heating, soaking, and cooling depends entirely on the intended application of the component.
- If your primary focus is maximum hardness and wear resistance (e.g., cutting tools): You will use a process defined by a very rapid quench.
- If your primary focus is maximum ductility and softness (e.g., for easy machining or forming): You will use a process defined by a very slow cooling rate, like annealing.
- If your primary focus is a balance of high strength and good toughness (e.g., structural bolts, gears): You will use a two-step quench-and-temper process to achieve the optimal compromise.
By precisely controlling these three thermal factors, you can engineer a material's properties to meet the exact demands of its function.
Summary Table:
| Factor | Key Role | Impact on Material Properties |
|---|---|---|
| Heating Rate & Temperature | Prevents thermal shock; reaches transformation temperature (e.g., austenitizing). | Ensures uniform structural change; avoids warping/cracking. |
| Soaking Time | Allows complete, uniform microstructural transformation throughout the part. | Guarantees consistent properties; prevents weak spots. |
| Cooling Rate | "Locks in" the final microstructure (e.g., martensite for hardness). | Directly determines final hardness, strength, and toughness. |
Ready to Achieve Precise Material Properties in Your Lab?
Mastering heat treatment is key to developing high-performance materials. KINTEK specializes in providing the precise, reliable lab equipment—from advanced furnaces to controlled quenching systems—that you need to consistently apply these critical thermal factors.
Whether you're working on R&D, quality control, or production, our solutions help you control temperature, time, and cooling with accuracy. Let's discuss your specific application and material goals.
Contact our experts today to optimize your heat treatment processes!
Visual Guide
Related Products
- Vacuum Heat Treat and Pressure Sintering Furnace for High Temperature Applications
- Graphite Vacuum Furnace Bottom Discharge Graphitization Furnace for Carbon Materials
- Horizontal High Temperature Graphite Vacuum Graphitization Furnace
- Large Vertical Graphite Vacuum Graphitization Furnace
- 1200℃ Muffle Furnace Oven for Laboratory
People Also Ask
- What is sputtering technology? A Guide to Precision Thin Film Deposition
- How does a sputtering machine work? Achieve Atomic-Level Precision for Your Coatings
- What is the purpose of using vacuum-sealed glass tubes for Thio-LISICON sintering? Optimize Solid Electrolyte Purity
- What are the stages of sintering? A Guide to Mastering the Powder-to-Part Process
- What is a sputtering machine? A Guide to High-Quality Thin Film Deposition