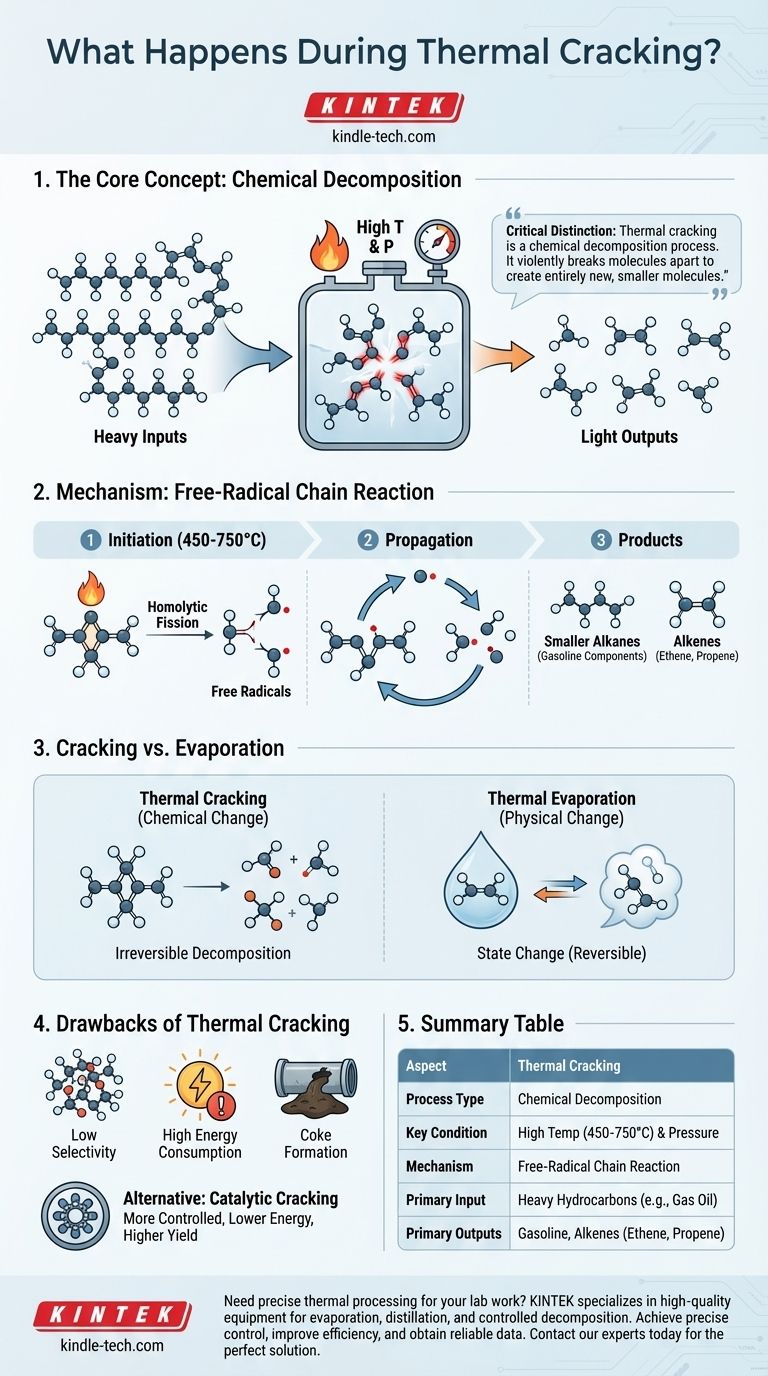
In essence, thermal cracking is a chemical process that uses high temperatures and pressures to break down large, complex hydrocarbon molecules into smaller, more valuable ones. Unlike a simple phase change like boiling, this process fundamentally alters the chemical structure of the molecules by cleaving their carbon-carbon bonds. The primary inputs are heavy, low-value hydrocarbon fractions, and the outputs are lighter, more useful products like gasoline components and alkenes.
The critical distinction to understand is that thermal cracking is a chemical decomposition process. It doesn't just change a substance's state (like melting or boiling); it violently breaks molecules apart to create entirely new, smaller molecules.

The Core Mechanism of Molecular Breakdown
Thermal cracking is not a gentle process. It relies on brute force—in the form of thermal energy—to initiate a chain reaction that shatters long-chain hydrocarbons.
Initiating the Reaction
The process begins by heating heavy hydrocarbon feedstocks, such as gas oil or naphtha, to very high temperatures, typically between 450°C and 750°C (842°F and 1382°F), under high pressure. This intense thermal energy provides the activation energy required to break the strong carbon-carbon single bonds within the large molecules.
The Free-Radical Chain Reaction
The initial breaking of a carbon-carbon bond is called homolytic fission. This event splits the bond evenly, creating two highly reactive fragments, each with an unpaired electron. These fragments are known as free radicals.
These unstable free radicals immediately attack other large hydrocarbon molecules, propagating a chain reaction that breaks them down into a variety of smaller molecules and more free radicals, continuing the cycle.
Key Products Formed
This process results in a mixture of smaller, more valuable products. The primary outputs are typically:
- Smaller Alkanes: These are used to increase the yield of high-octane gasoline.
- Alkenes: Molecules like ethene (ethylene) and propene (propylene) are crucial building blocks for the petrochemical industry, used to produce polymers and plastics.
Distinguishing Cracking from Other Thermal Processes
The term "thermal" can cause confusion, as it applies to many different scientific processes. Understanding the fundamental difference between a chemical change and a physical change is key.
Thermal Cracking vs. Thermal Evaporation
Thermal cracking induces a chemical change. Its purpose is to break molecular bonds and create entirely new substances. This is an irreversible decomposition.
Thermal evaporation, on the other hand, is a physical change. It uses heat to turn a solid or liquid into a vapor for applications like thin-film deposition. The molecules themselves remain intact; they just transition into a gaseous state.
Catalytic Cracking: A More Controlled Alternative
Modern refineries often prefer catalytic cracking. This process achieves the same molecular breakdown but uses a catalyst to lower the required temperature and pressure. This provides greater control over the reaction, leading to a higher yield of desired products and less energy consumption.
Understanding the Trade-offs and Limitations
While effective, traditional thermal cracking has several significant drawbacks that have led to the adoption of more advanced methods.
Lack of Selectivity
The free-radical mechanism is difficult to control. It produces a broad mixture of hydrocarbons, and the yield of the specific desired product can be lower than with more targeted methods.
High Energy Consumption
Achieving and maintaining the extreme temperatures and pressures required for thermal cracking is a very energy-intensive and expensive process.
Coke Formation
A common and problematic side effect is the formation of a solid, carbon-rich residue known as coke. This material deposits on the reactor walls, reducing efficiency and requiring periodic shutdowns for removal.
How This Applies in Practice
Choosing or understanding a thermal process depends entirely on your objective—whether you need to break molecules apart or simply change their state.
- If your primary focus is producing high-octane gasoline efficiently: Modern refineries almost exclusively use fluid catalytic cracking (FCC) for its superior control and higher-quality output.
- If your primary focus is producing foundational alkenes (ethene/propene): A specific high-temperature form of thermal cracking called "steam cracking" remains the dominant industrial method for this purpose.
- If your primary focus is changing a material's state without altering its chemistry: You are looking for a physical process like thermal evaporation or distillation, not a chemical process like cracking.
Understanding the fundamental difference between breaking chemical bonds and changing physical states is the key to mastering these essential industrial processes.
Summary Table:
| Aspect | Thermal Cracking |
|---|---|
| Process Type | Chemical Decomposition |
| Key Condition | High Temperature (450-750°C) & Pressure |
| Mechanism | Free-Radical Chain Reaction |
| Primary Input | Heavy Hydrocarbons (e.g., Gas Oil) |
| Primary Outputs | Gasoline, Alkenes (e.g., Ethene, Propene) |
Need precise thermal processing for your lab work?
Whether you're developing new materials or analyzing hydrocarbon samples, having the right equipment is crucial for achieving accurate and repeatable results. Thermal processes like evaporation, distillation, and controlled decomposition are fundamental to laboratory success.
At KINTEK, we specialize in high-quality lab equipment and consumables designed to meet the rigorous demands of modern laboratories. Our range of thermal processing equipment can help you:
- Achieve precise temperature control for your experiments.
- Improve the efficiency and safety of your processes.
- Obtain reliable and consistent data.
Let KINTEK be your partner in innovation. Contact our experts today to find the perfect solution for your specific thermal application needs.
Visual Guide
Related Products
- Vertical Laboratory Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
People Also Ask
- What is the alternative to a tube furnace? A Guide to Choosing the Right Heating Tool for Your Lab
- Why is a tube reduction furnace required for Fe-Cu powders? Eliminate Oxides for Superior Sintering Results
- What are the functions of a high-pressure horizontal tube furnace in 650 °C CO2 oxidation experiments?
- What technical considerations lead to the selection of a quartz tube reactor for methane steam reforming (MSR)?
- What is the primary function of the vacuum tube furnace in the preparation process of ZnS nanopowder? (800°C Calcination)
- Why is a high-temperature vertical tube furnace required for MOE? Ensure Precise 1600°C Oxygen Validation
- What is the process of annealing tubes? Achieve Optimal Softness and Ductility for Your Tubing
- What is the primary advantage of using a tube furnace? Achieve Superior Temperature and Atmosphere Control



















