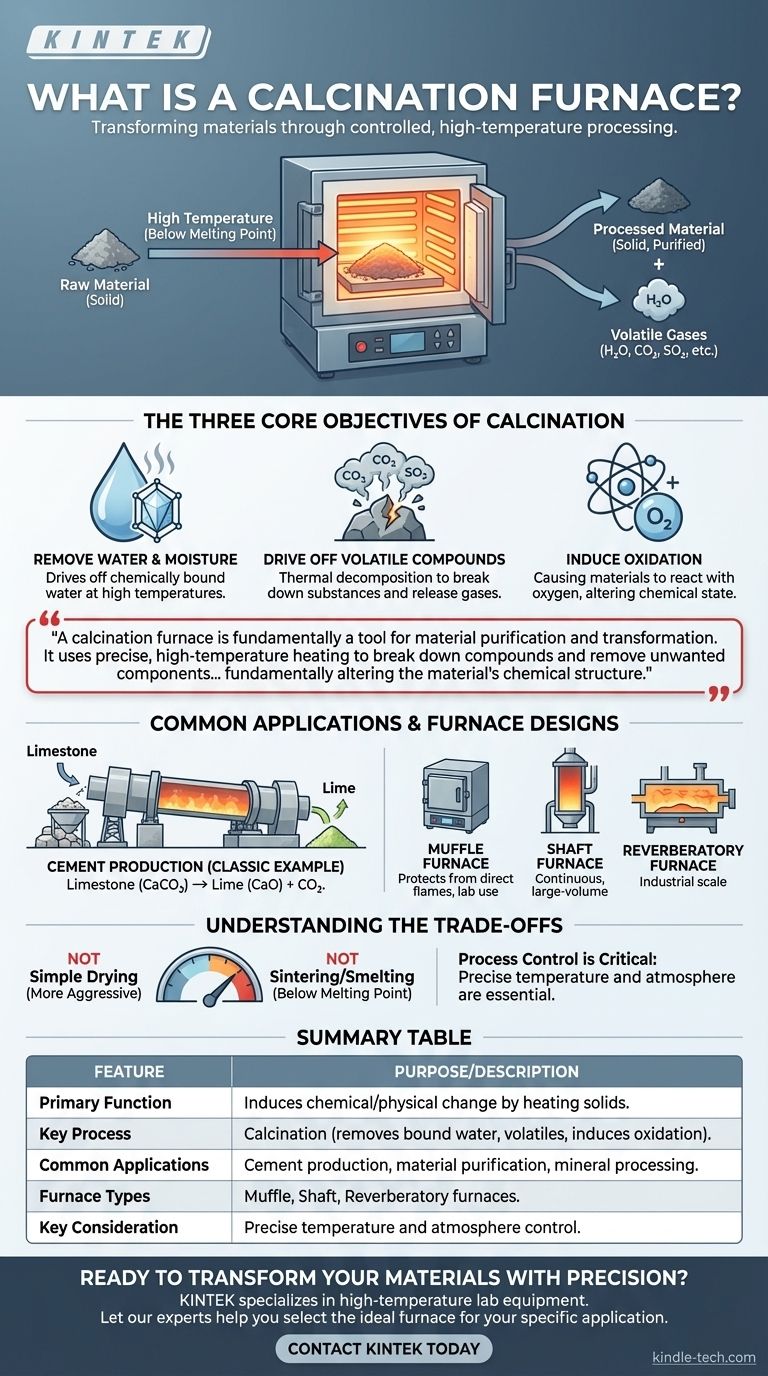
In essence, a calcination furnace is a high-temperature industrial oven designed to heat solid materials to induce a specific chemical reaction or physical change. Its primary purpose is not merely to heat something, but to transform it by removing volatile substances, driving off absorbed moisture, or deliberately causing oxidation.
A calcination furnace is fundamentally a tool for material purification and transformation. It uses precise, high-temperature heating to break down compounds and remove unwanted components like water, carbon dioxide, or other gases, fundamentally altering the material's chemical structure.

The Three Core Objectives of Calcination
The process of calcination is defined by its outcome. A furnace is engineered to achieve one or more of these specific goals with a high degree of control.
Removing Water and Moisture
This goes beyond simple drying. Calcination is used to drive off water that is chemically bound within a material's crystal structure, which requires significantly higher temperatures than evaporation.
Driving Off Volatile Compounds
This is the most common objective, involving the thermal decomposition of a material. The heat causes the substance to break down, releasing gases and leaving a solid product behind.
A prime example is removing carbon dioxide (CO2) from limestone or sulfur dioxide (SO2) from certain minerals.
Inducing Oxidation
In some applications, the furnace is used to cause a material to react with oxygen in the air. This process, known as oxidation, can be used to convert a substance from one chemical state to another.
Common Applications and Furnace Designs
While the principles are straightforward, the applications and equipment can vary significantly depending on the industrial scale and desired outcome.
Cement Production: The Classic Example
The most widespread use of calcination is in producing cement. In massive kilns, calcium carbonate (limestone) is heated to decompose it into calcium oxide (lime) and carbon dioxide gas. The resulting lime is a primary ingredient in cement.
Common Furnace Configurations
Calcination furnaces are not one-size-fits-all. The design is chosen based on the material being processed and the required throughput.
Common types include muffle furnaces, which protect the material from direct flames, and industrial-scale shaft furnaces or reverberatory furnaces, which are built for continuous, large-volume production.
Understanding the Trade-offs
It is crucial to distinguish calcination from other thermal processes. It is a more aggressive and transformative process than simple drying but is typically done at temperatures below the material's melting point, unlike sintering or smelting.
Process Control is Critical
The temperature and atmosphere inside the furnace must be precisely controlled. Insufficient heat will result in an incomplete reaction, while excessive heat could damage the material or cause unintended chemical changes.
Not a Universal Solution
Calcination is specifically for driving off volatile components or inducing oxidation. If the goal is simply to melt a material or fuse particles together without chemical change, a different type of furnace and process is required.
Applying This to Your Goal
Choosing the right thermal process depends entirely on the desired end-state of your material.
- If your primary focus is simple moisture removal: A standard industrial drying oven may be sufficient and more energy-efficient.
- If your primary focus is inducing a chemical breakdown: A calcination furnace is the correct instrument, with the specific design depending on your production scale.
- If your primary focus is changing the material's crystal structure or purity: Calcination is the necessary step to remove volatile impurities and prepare the material for further processing.
Ultimately, a calcination furnace is a powerful instrument for fundamentally altering the chemical composition of solid materials through controlled heat.
Summary Table:
| Feature | Purpose/Description |
|---|---|
| Primary Function | Induces chemical/physical change by heating solids to high temperatures. |
| Key Process | Calcination (removes bound water, volatiles like CO2, or induces oxidation). |
| Common Applications | Cement production (limestone to lime), material purification, mineral processing. |
| Common Furnace Types | Muffle furnaces, shaft furnaces, reverberatory furnaces. |
| Key Consideration | Requires precise temperature and atmosphere control for a complete reaction. |
Ready to transform your materials with precision?
Whether your goal is material purification, driving off volatile compounds, or preparing samples for further analysis, the right calcination furnace is critical. KINTEK specializes in high-temperature lab equipment, including robust calcination furnaces designed for accuracy and reliability in research and quality control.
Let our experts help you select the ideal furnace for your specific application.
Contact KINTEL today to discuss your laboratory's calcination needs and discover how our solutions can enhance your processes.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1800℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- What is the process of a muffle furnace? From Electricity to Precision High-Temp Control
- What is the yield of biochar in slow pyrolysis? Maximize Your Output Up to 30%
- What is the precaution of furnace? Essential Safety Steps to Protect Operators and Equipment
- What are the five common heat treatments of metals? Master the Processes for Precise Material Properties
- What is the difference between muffle furnace and tubular furnace? A Guide to Choosing the Right Lab Furnace



















