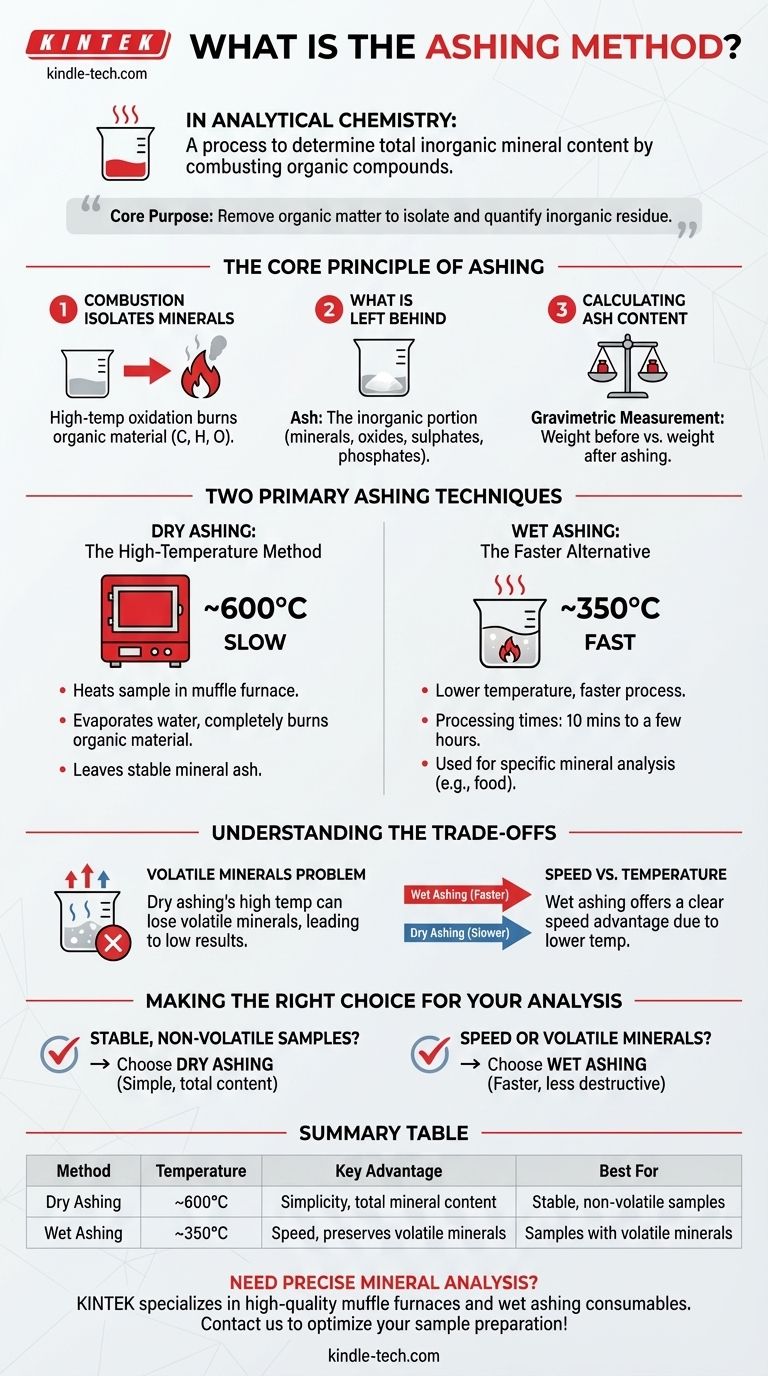
In analytical chemistry, the ashing method is a process used to determine the total amount of inorganic mineral content within a sample. It works by heating the sample at a high temperature in the presence of air, causing all the organic compounds to combust and turn into gases. The material left behind is the non-combustible, inorganic ash, which represents the total mineral content.
The core purpose of ashing is to remove all organic matter from a sample to isolate and quantify the inorganic residue. The choice between the primary methods, dry or wet ashing, depends on the sample's characteristics and the need to preserve volatile minerals.

The Core Principle of Ashing
How Combustion Isolates Minerals
The fundamental process relies on high-temperature oxidation. When a prepared sample is heated, the organic material—compounds primarily made of carbon, hydrogen, and oxygen—reacts with oxygen in the air and burns away.
What is Left Behind
The remaining substance, or ash, is the inorganic portion of the sample that does not combust. This residue consists of minerals that are transformed into more stable forms like oxides, sulphates, and phosphates.
Calculating Ash Content
The analysis is a form of gravimetric measurement. The percentage of ash content is calculated based on the difference in the weight of the sample before and after the ashing process is complete.
Two Primary Ashing Techniques
Dry Ashing: The High-Temperature Method
Dry ashing is a common technique that involves heating a sample in a high-temperature muffle furnace, typically around 600°C.
At this temperature, any water is first driven off through evaporation. The remaining organic material is then completely burned down, leaving only the stable mineral ash.
Wet Ashing: The Faster Alternative
Wet ashing is another widely used method, especially for food samples, that operates at a lower temperature of approximately 350°C.
This technique is significantly faster than dry ashing, with processing times ranging from just 10 minutes to a few hours. It is used to prepare samples for the analysis of specific minerals.
Understanding the Trade-offs
The Problem of Volatile Minerals
The primary drawback of dry ashing is its high temperature. If the sample contains volatile minerals, they can be lost during the process, leading to an inaccurate and artificially low measurement of the total ash content.
Speed vs. Temperature
Wet ashing offers a clear advantage in speed. Its lower operating temperature makes it a faster process from start to finish.
Choosing Based on Sample Composition
The most critical factor in choosing a method is the nature of the sample itself. The presence of volatile materials makes the high heat of a muffle furnace a significant liability for accurate analysis.
Making the Right Choice for Your Analysis
Choosing the correct ashing method is essential for obtaining accurate and reliable data on the mineral content of your sample.
- If your primary focus is analyzing stable, non-volatile samples: Dry ashing is a straightforward and effective method for determining total mineral content.
- If your primary focus is speed or analyzing samples with potentially volatile minerals: Wet ashing is the superior choice due to its faster processing time and lower, less destructive temperature.
Ultimately, understanding the composition of your sample is the key to selecting the correct ashing method for accurate mineral analysis.
Summary Table:
| Method | Temperature | Key Advantage | Best For |
|---|---|---|---|
| Dry Ashing | ~600°C | Simplicity, total mineral content | Stable, non-volatile samples |
| Wet Ashing | ~350°C | Speed, preserves volatile minerals | Samples with volatile minerals |
Need precise mineral analysis in your lab? Choosing the right ashing method is critical for accurate results. KINTEK specializes in high-quality lab equipment, including reliable muffle furnaces for dry ashing and consumables for wet ashing. Our experts can help you select the perfect solution for your laboratory's specific needs. Contact us today to optimize your sample preparation process!
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- What is the alternative to a laboratory oven? Find the Right Heating Tool for Your Lab
- What is the difference between a muffle furnace and a chamber furnace? Understand the Key Distinctions for Your Lab
- What is a muffle furnace used in determination of? Precise Ash Content and Material Composition
- How do you calibrate a muffle furnace? Achieve Precise Temperature Control for Your Lab
- What is the heating mechanism of a muffle furnace? Achieve Clean, Uniform High-Temperature Processing



















