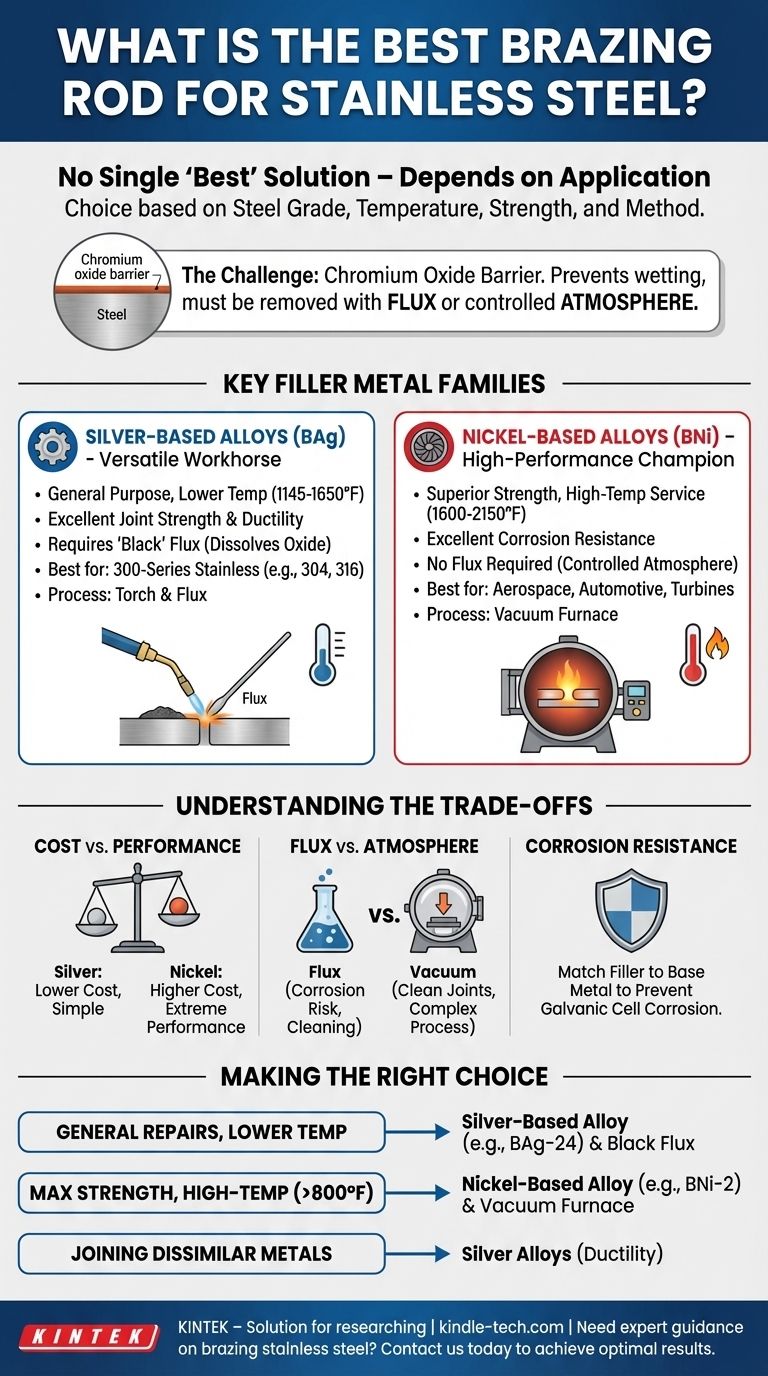
To be direct, there is no single "best" brazing rod for all stainless steel applications. The ideal choice depends on the specific grade of stainless steel, the required service temperature, strength requirements, and the brazing method you plan to use. However, the most common and effective choices fall into two main families: silver-based alloys for general-purpose, lower-temperature work, and nickel-based alloys for high-performance, high-temperature applications.
The challenge of brazing stainless steel stems from its protective chromium oxide layer, which must be managed. The "best" filler metal is the one that not only creates a strong bond but also has properties—like temperature resistance and corrosion resistance—that are compatible with the base metal and the final application.

Why Brazing Stainless Steel is a Unique Challenge
The very property that makes stainless steel "stainless"—a thin, invisible, and self-healing layer of chromium oxide—is the primary obstacle in brazing.
The Chromium Oxide Barrier
For a brazing alloy to bond with a metal, it must be able to "wet" the surface, meaning it must flow cleanly and adhere to the pure metal underneath.
The chromium oxide layer prevents this wetting. Therefore, any successful stainless steel brazing operation must first either chemically remove this layer with flux or prevent it from forming in the first place using a controlled atmosphere.
Key Filler Metal Families for Stainless Steel
Your choice of filler metal, or "brazing rod," is dictated by the demands of the job.
Silver-Based Alloys (The Versatile Workhorse)
Silver brazing alloys are the most common choice for a wide range of stainless steel applications, particularly for the 300-series austenitic grades (e.g., 304, 316).
These alloys, often designated as BAg grades, are valued for their relatively low brazing temperatures (1145-1650°F / 618-899°C). This lower heat input reduces the risk of warping the part or negatively affecting the steel's inherent corrosion resistance. They provide excellent joint strength and ductility.
When using silver alloys, a brazing flux is almost always required to dissolve the chromium oxide layer and protect the joint during heating. For stainless steel, a "black" flux is necessary as it remains active at the higher temperatures required compared to flux for copper or brass.
Nickel-Based Alloys (The High-Performance Champion)
For applications demanding superior strength, high-temperature service, or maximum corrosion resistance, nickel-based filler metals are the standard.
These alloys, designated as BNi grades, have much higher brazing temperatures (1600-2150°F / 871-1177°C). The resulting joints can withstand extreme operating environments found in aerospace, automotive turbochargers, and industrial turbines.
Due to these high temperatures, BNi alloys are not used with flux. Instead, they are used exclusively in controlled atmosphere furnaces, most commonly a vacuum furnace. As noted in the reference, this process pulls a vacuum to remove all oxygen, preventing oxides from ever forming and resulting in exceptionally clean, strong joints without any flux residue.
Understanding the Trade-offs
Choosing a filler metal is an exercise in balancing performance, process complexity, and cost.
Cost vs. Performance
Silver alloys are generally less expensive than nickel alloys and can be applied with a simple torch and flux.
Nickel alloys are more costly and require significant capital investment in a vacuum or atmosphere furnace. However, their performance in high-stress, high-temperature environments is unmatched.
Flux vs. Atmosphere
Using flux is effective but introduces a potential failure point. If flux becomes trapped in the joint, it can lead to corrosion over time. The post-braze cleaning required to remove flux residue also adds another step to the process.
Vacuum brazing entirely eliminates flux, producing cleaner joints with superior integrity, but the process is far less accessible and more expensive than open-air torch brazing.
Matching Corrosion Resistance
A critical consideration is ensuring the filler metal's corrosion resistance is compatible with the stainless steel base metal. Using a less-resistant filler can create a galvanic cell, where the joint corrodes preferentially when exposed to an electrolyte, leading to premature failure.
Making the Right Choice for Your Application
Select your filler metal based on the functional requirements of the finished part.
- If your primary focus is general-purpose repairs or joining 300-series stainless at lower temperatures: A silver-based alloy (like BAg-24) applied with a black brazing flux is your most practical and effective choice.
- If your primary focus is maximum joint strength and high-temperature service (above 800°F / 425°C): A nickel-based alloy (like BNi-2) used within a vacuum furnace is the correct engineering solution.
- If your primary focus is joining dissimilar metals, one of which is stainless steel: Silver alloys are often preferred for their ductility and ability to bridge the different expansion rates of the two metals.
By matching the filler metal and process to the specific demands of the stainless steel alloy, you ensure a joint with uncompromising strength and reliability.
Summary Table:
| Filler Metal Type | Best For | Brazing Temperature | Key Process |
|---|---|---|---|
| Silver-Based (BAg) | General-purpose, lower-temperature applications (e.g., 304, 316 stainless) | 1145-1650°F (618-899°C) | Requires flux (e.g., 'black' flux) |
| Nickel-Based (BNi) | High-performance, high-temperature applications (e.g., aerospace, turbines) | 1600-2150°F (871-1177°C) | Requires controlled atmosphere (e.g., vacuum furnace) |
Need expert guidance on brazing stainless steel for your specific application?
KINTEK specializes in providing advanced brazing solutions and equipment for laboratories and industrial settings. Whether you're working with silver-based alloys for general repairs or require nickel-based alloys and vacuum furnaces for high-temperature performance, our team can help you select the right materials and processes to ensure strong, durable, and corrosion-resistant joints.
Contact us today to discuss your brazing needs and achieve optimal results with KINTEK's expertise in lab equipment and consumables!
Visual Guide
Related Products
- Stainless Steel Quick Release Vacuum Chain Three-Section Clamp
- Custom PTFE Teflon Parts Manufacturer for Gaskets and More
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Custom PTFE Teflon Parts Manufacturer for PTFE Bottle Oil Fume Sampling Tube
- Laboratory Rotary Vane Vacuum Pump for Lab Use
People Also Ask
- Why must a laboratory vacuum pump be used to evacuate a PM-HIP capsule before it is sealed? Ensure Material Integrity
- What environmental protection do mechanical vacuum pump sets provide during zirconium alloy melting? Prevent Embrittlement
- How can a gas ballast valve be used as a diagnostic tool? Identify Oil Contamination vs. System Leaks
- What is the function of a high-temperature vacuum annealing furnace? Optimize Your Zr2Al3C4 Coating Formation
- What is vacuum melt steel? Unlock Unmatched Purity and Performance for Critical Applications















