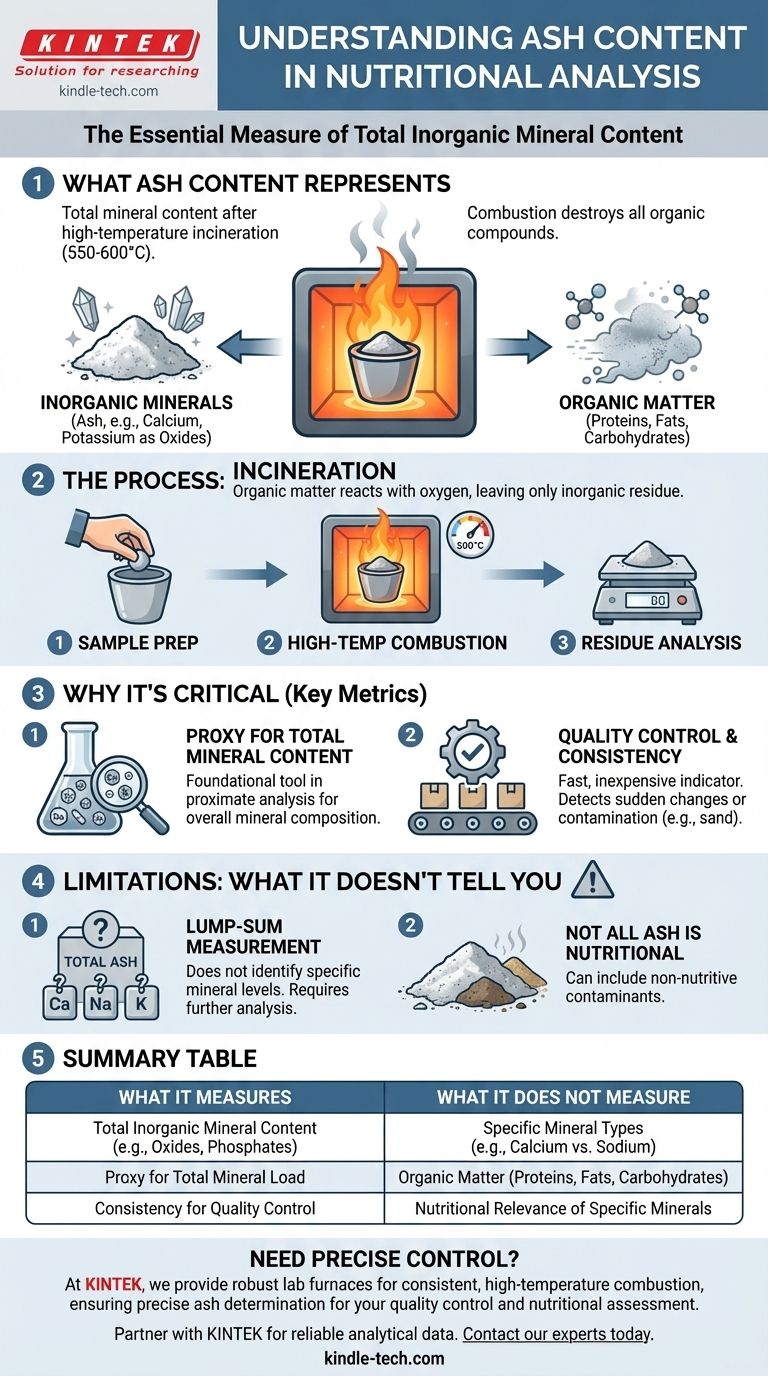
In nutritional analysis, ash content is the most common measure of the total mineral content within a food, feed, or biological sample. When a sample is subjected to high-temperature combustion, all the organic matter (like proteins, fats, and carbohydrates) burns away, leaving behind only the inorganic, noncombustible materials, which are the minerals.
Ash analysis provides a single value representing the total quantity of minerals in a sample. While it is an essential metric for quality control and overall nutritional assessment, it does not identify which specific minerals are present.

What "Ash" Actually Represents
To understand ash content, you must understand the process used to measure it. The analysis physically separates the organic and inorganic components of a sample.
The Role of Combustion
The process, known as incineration or ashing, involves placing a sample in a furnace at extremely high temperatures, typically between 550 and 600°C.
At this temperature, all organic compounds—which are based on carbon—react with oxygen and are destroyed. This includes proteins, fats, carbohydrates, and most vitamins.
The Inorganic Residue
What remains after complete combustion is a small amount of whitish-gray residue, or "ash." This ash is composed of the inorganic elements that were present in the original sample.
These elements exist as oxides, sulfates, phosphates, chlorides, or silicates. For example, calcium becomes calcium oxide, and potassium becomes potassium chloride.
Why Measuring Ash is a Critical Metric
While simple, the ash test is a foundational tool in food science, quality control, and animal nutrition for several key reasons.
A Proxy for Total Mineral Content
Ash content serves as a direct proxy for the total mineral load of a product. It gives a single, quantitative value that helps formulators and analysts understand the overall mineral composition.
This is a fundamental part of a proximate analysis, a standard set of tests that profiles a food into its major components: moisture, ash, crude protein, crude fat, and crude fiber.
A Tool for Quality Control
In manufacturing, ash content is a fast and inexpensive indicator of product consistency. A sudden change in the ash value of a finished product can signal a problem in the raw ingredients or the production process.
For instance, an unusually high ash content in an animal feed could indicate contamination with sand or soil, which adds weight but has no nutritional value.
Understanding the Limitations
While invaluable, it's crucial to recognize what ash content does not tell you. Relying on it without understanding its limitations can lead to incorrect conclusions.
It Doesn't Identify Specific Minerals
The single greatest limitation is that ash content is a lump-sum measurement. It tells you the total amount of minerals but provides no information about the levels of specific, individual minerals.
A food could have a healthy ash content but be deficient in a critical mineral like calcium and excessively high in another, like sodium. Further analysis (e.g., atomic absorption spectroscopy) is required to determine the specific mineral profile.
Not All Ash is Nutritionally Relevant
The ash value includes all inorganic matter, not just the nutritionally essential minerals. It can include contaminants like soil, sand, or other non-nutritive inorganic additives.
Therefore, a high ash value is not automatically "better." It must be interpreted in the context of the expected value for that specific ingredient or product.
How to Interpret Ash Content in Your Analysis
Your interpretation of an ash value depends entirely on your goal. It can be a quality check, a nutritional starting point, or a component of a larger calculation.
- If your primary focus is quality control: Use ash content as a rapid and reliable check to verify product consistency and screen for inorganic adulterants like sand or dirt.
- If your primary focus is nutritional formulation: View the ash value as the first step for confirming the total mineral package is within specification before conducting more expensive tests for specific minerals like calcium, phosphorus, or potassium.
- If your primary focus is general food analysis: Recognize that ash is a core part of proximate analysis, which is essential for calculating total carbohydrate content and the overall caloric value of a food product.
Ultimately, understanding ash content is a fundamental skill for anyone involved in evaluating the quality and nutritional composition of food and feed.
Summary Table:
| Aspect | What Ash Content Measures | What It Does Not Measure |
|---|---|---|
| Nutrient Focus | Total inorganic mineral content | Specific mineral types (e.g., Calcium, Sodium) |
| Composition | Oxides, phosphates, chlorides of minerals | Organic matter (proteins, fats, carbohydrates) |
| Primary Use | Quality control, proximate analysis, nutritional screening | Detailed mineral profiling or identifying contaminants |
Need precise control over your nutritional analysis?
At KINTEK, we understand that accurate ash content measurement is the foundation of reliable quality control and nutritional assessment. Our robust lab furnaces and consumables are designed to deliver consistent, high-temperature combustion for precise ash determination, ensuring your product quality and consistency.
Whether you're in food science, feed production, or agricultural research, partnering with KINTEK provides you with the reliable equipment needed to build trust in your analytical data.
Contact our experts today to find the perfect ashing solution for your laboratory's needs.
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is a muffle furnace used for? Achieve Pure, High-Temperature Processing
- What is a muffle furnace in the environment? Achieve Clean, Contaminant-Free Heating
- What is the maximum temperature of a muffle furnace? Find the Right Heat for Your Application
- What is a muffle furnace used in determination of? Precise Ash Content and Material Composition
- What is the temperature accuracy of a muffle furnace? Achieve Precise and Uniform Heating



















