The extraordinary strength of carbon nanotubes stems directly from the unique nature and arrangement of their carbon-carbon bonds. These sp² covalent bonds are the strongest type of chemical bond in nature, and within a nanotube, they form a seamless, near-perfect hexagonal lattice. This flawless cylindrical structure distributes stress evenly across the entire molecule, allowing it to withstand immense tensile forces without breaking.
The core reason for a carbon nanotube's strength is not just the inherent power of its atomic bonds, but the perfection of its molecular structure. It's a case where the whole is truly stronger than the sum of its parts because there are virtually no weak points to initiate failure.
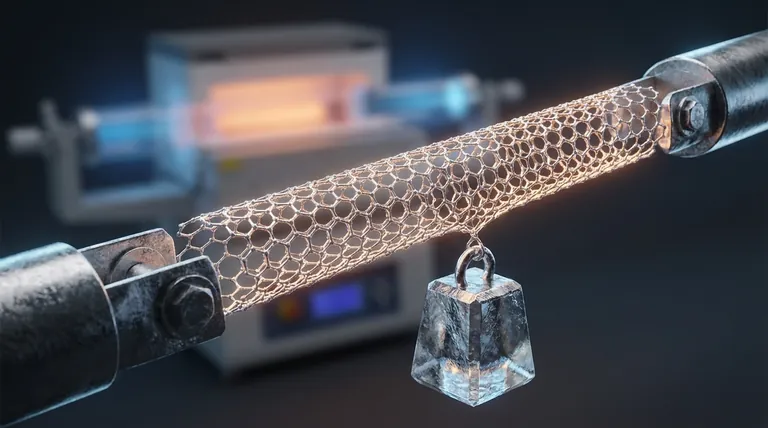
The Atomic Foundation: sp² Hybridization
The story of carbon nanotube (CNT) strength begins with the way each carbon atom connects to its neighbors. This specific bonding configuration is the ultimate source of its remarkable mechanical properties.
The Power of the Sigma (σ) Bond
In a nanotube, each carbon atom is bonded to three other carbon atoms. It uses its outer electrons to form three sp² hybridized orbitals.
These orbitals arrange themselves in a flat plane, 120 degrees apart, and form incredibly strong sigma (σ) bonds with neighboring atoms. A sigma bond is a direct, head-on overlap of orbitals, creating the most stable and robust type of covalent link possible.
A Seamless Hexagonal Lattice
These sigma bonds create the familiar honeycomb-like hexagonal lattice that makes up the wall of the nanotube. Think of it as a rolled-up sheet of graphene.
This structure is supremely efficient at distributing any applied force. When you pull on a nanotube, the stress is shared equally across millions of these ultra-strong bonds, with no single point bearing the entire load.
The Role of Pi (π) Bonds
The remaining unhybridized p-orbital on each carbon atom forms weaker pi (π) bonds that are delocalized across the entire surface of the tube. While these bonds are responsible for the nanotube's high electrical conductivity, it is the underlying framework of sigma bonds that provides its record-breaking strength.
From Atomic Bonds to Macroscopic Strength
A strong bond is only one part of the equation. The arrangement of those bonds into a macroscopic structure is what translates atomic potential into real-world performance.
The Near-Perfect Crystalline Structure
Most engineering materials, like steel or aluminum, are polycrystalline. They are composed of countless tiny crystal grains with boundaries between them. These grain boundaries, along with other microscopic voids and dislocations, are inherent weak points where cracks can begin.
An ideal carbon nanotube, by contrast, is a single, continuous molecule. It has no grain boundaries. This lack of defects means that its practical strength can approach its theoretical strength, which is governed only by the force required to break the carbon-carbon bonds themselves.
High Aspect Ratio and Load Transfer
Carbon nanotubes possess an extremely high aspect ratio, meaning they are exceptionally long relative to their diameter.
This property is critical for their use in composite materials. A long nanotube can effectively bridge microcracks in a surrounding polymer or ceramic matrix, transferring the load along its entire length and preventing the material from failing.
Understanding the Real-World Limitations
The incredible strength values often cited for CNTs—up to 100 times stronger than steel at a fraction of the weight—apply to individual, perfect nanotubes measured under ideal laboratory conditions. Harnessing this strength in bulk materials presents significant challenges.
Theoretical vs. Practical Strength
Real-world synthesis methods inevitably introduce defects into the nanotube's lattice. A single missing atom (a vacancy) or a misaligned bond can act as a stress concentration point, dramatically reducing the tube's tensile strength.
The Problem of Agglomeration
Due to weak attractive forces known as van der Waals forces, individual nanotubes have a strong tendency to clump together into bundles. These clumps are incredibly difficult to separate.
When mixed into a composite, these bundles act like weak inclusions rather than strong reinforcements, as the tubes simply slide past one another instead of bearing the load. Achieving proper dispersion is a primary challenge in CNT composites.
Weak Interfacial Bonding
For a nanotube to reinforce a material, stress must be efficiently transferred from the host material (the matrix) to the nanotube. This requires a strong interfacial bond.
Often, the chemical interaction between the nanotube surface and the matrix is weak. If this interface fails before the nanotube does, the composite gains little to no strength benefit.
Applying This Knowledge to Your Goal
Understanding the source of a CNT's strength—and its limitations—is key to leveraging it effectively. Your approach will depend entirely on your objective.
- If your primary focus is computational modeling: Your work should center on the perfect sp² sigma bond framework and the flawless hexagonal lattice to establish theoretical performance limits.
- If your primary focus is composite material development: Your main challenges are overcoming agglomeration to achieve uniform dispersion and engineering strong interfacial adhesion between the CNTs and the matrix.
- If your primary focus is CNT synthesis: Your goal is to refine growth processes to minimize atomic defects and produce longer, more structurally perfect nanotubes that can realize their intrinsic strength.
Ultimately, the power of a carbon nanotube is a direct lesson in how atomic-level design dictates macroscopic performance.
Summary Table:
| Key Factor | Contribution to Strength |
|---|---|
| sp² Hybridization | Forms ultra-strong sigma (σ) covalent bonds between carbon atoms. |
| Seamless Hexagonal Lattice | Distributes stress evenly across the entire structure, eliminating weak points. |
| Single-Molecule Structure | Lack of grain boundaries allows strength to approach theoretical limits. |
| High Aspect Ratio | Enables effective load transfer in composite materials by bridging microcracks. |
Ready to harness the power of advanced materials in your lab?
Understanding the atomic-level strength of carbon nanotubes is just the beginning. At KINTEK, we specialize in providing the high-quality lab equipment and consumables you need to turn this knowledge into real-world results. Whether you are developing next-generation composite materials, refining synthesis processes, or conducting precise material testing, our products are designed to support your most demanding research and development goals.
Let's build the future of materials science together. Contact our experts today to discuss how KINTEK can equip your laboratory for success.
Visual Guide

Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Multi-zone Laboratory Tube Furnace
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- Vertical Laboratory Tube Furnace
People Also Ask
- What role does Chemical Vapor Deposition (CVD) equipment play in the preparation of C/C composites? Expert Analysis
- What are the methods of producing CNT? Scalable CVD vs. High-Purity Lab Techniques
- What is a CVD tube furnace? A Complete Guide to Thin-Film Deposition
- How high of temperature do carbon nanotubes in air have the ability to sustain? Understanding the Oxidation Limit
- What is the floating catalyst method? A Guide to High-Yield CNT Production



















