The relationship between heat treatment and corrosion resistance is complex and highly conditional. A correctly applied heat treatment can dramatically improve a material's ability to resist corrosion by optimizing its internal structure. However, an incorrect process can create new vulnerabilities, making the material more susceptible to corrosive attack than it was in its original state.
Heat treatment is not a universal corrosion-proofing tool. It is a precise metallurgical lever that modifies a material's microstructure, and this change can either fortify its defenses against corrosion or, if done improperly, create the very pathways for failure.

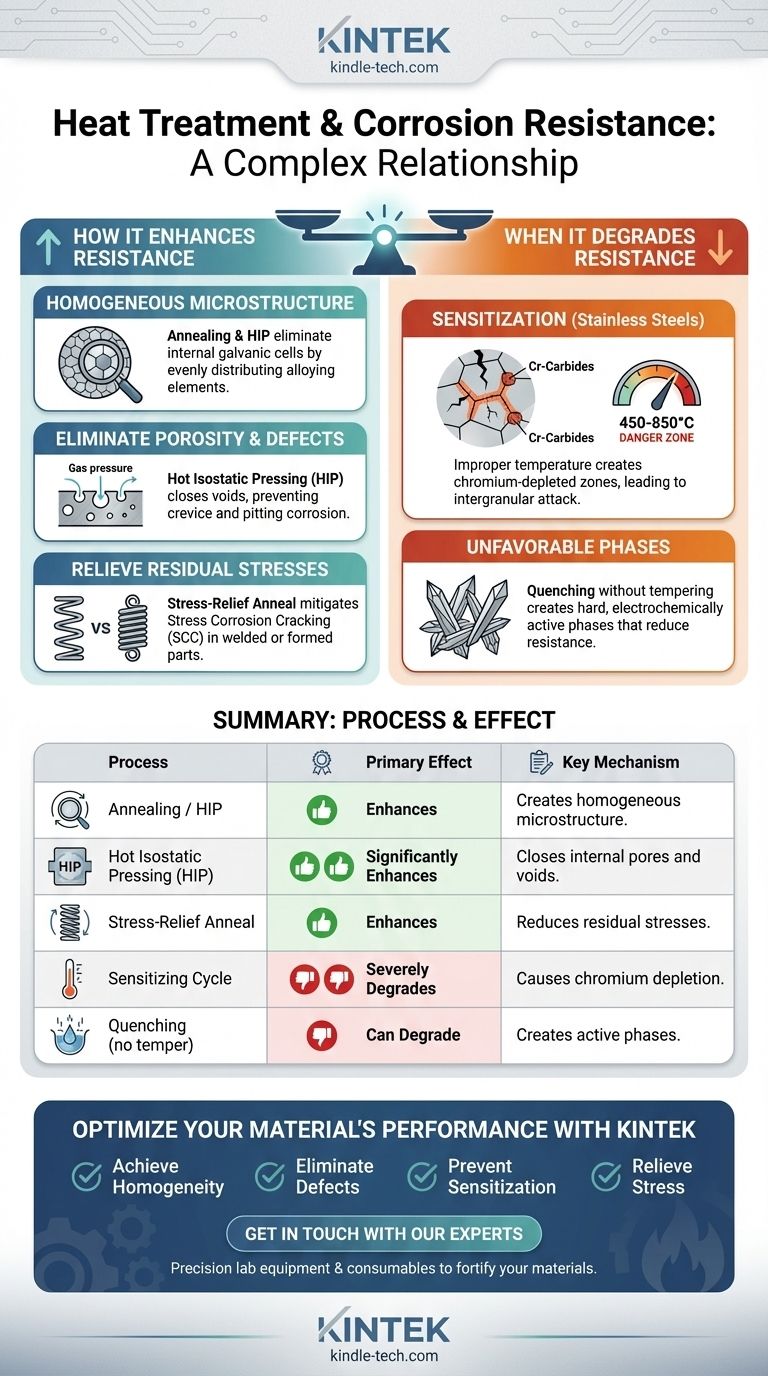
How Heat Treatment Can Enhance Corrosion Resistance
The primary positive effect of heat treatment is its ability to create a more uniform and stable microstructure. This directly hinders the chemical and electrochemical processes that define corrosion.
Creating a Homogeneous Microstructure
A material with a non-uniform chemical composition contains microscopic regions with different electrochemical potentials. When exposed to an electrolyte, these regions form tiny galvanic cells, accelerating localized corrosion.
Processes like annealing and Hot Isostatic Pressing (HIP) help to evenly distribute alloying elements, creating a chemically homogeneous structure. This eliminates the internal galvanic cells, significantly improving general corrosion resistance.
Eliminating Porosity and Defects
Microscopic pores, voids, and cracks from manufacturing processes like casting or powder metallurgy act as initiation sites for crevice corrosion and pitting.
Hot Isostatic Pressing (HIP), which combines high heat and isostatic gas pressure, physically closes these internal voids. This creates a fully dense material, removing the geometric traps where corrosive agents can concentrate and attack the material.
Relieving Residual Stresses
Mechanical processes like bending, forming, or welding leave residual stresses locked within the material. These high-energy areas are more chemically reactive and are prime targets for Stress Corrosion Cracking (SCC).
A stress-relief anneal is a low-temperature heat treatment specifically designed to reduce these internal stresses without significantly changing the material's hardness. This makes the material far more resistant to SCC failures.
Understanding the Trade-offs: When Heat Treatment Degrades Corrosion Resistance
While often beneficial, heat treatment can be a double-edged sword. Certain thermal cycles can inadvertently create microstructural features that severely compromise a material's integrity.
The Critical Pitfall: Sensitization in Stainless Steels
This is the most well-known negative effect of improper heat treatment. When austenitic stainless steels (like 304 or 316) are held within a specific temperature range (roughly 450-850°C or 840-1560°F), a detrimental change occurs.
Chromium atoms combine with carbon and precipitate as chromium carbides along the grain boundaries. This process strips the chromium from the adjacent areas, creating chromium-depleted zones. Since chromium is the key element providing corrosion resistance, these depleted zones become highly susceptible to intergranular corrosion.
Creating Unfavorable Phases
Heat treatments like quenching are designed to create very hard microstructures, such as martensite in steels. While excellent for strength and wear resistance, these highly-strained, non-equilibrium phases can be more electrochemically active and less corrosion-resistant than softer, more stable phases like ferrite or pearlite.
This creates a classic engineering trade-off where you must balance the need for mechanical properties against the demands of the corrosive environment. A tempering cycle is often required after quenching to regain some toughness and corrosion resistance at the expense of peak hardness.
Making the Right Choice for Your Application
The effect of heat treatment on corrosion is not a simple "good" or "bad" question. It is entirely dependent on the alloy, the process, and your final goal.
- If your primary focus is maximizing corrosion resistance in critical components: Consider advanced processes like Hot Isostatic Pressing (HIP) to create a dense, defect-free, and homogeneous microstructure.
- If you are working with austenitic stainless steels (e.g., 304, 316): Be vigilant about the time-temperature profile during heat treatment or welding to avoid the sensitization range that depletes chromium at the grain boundaries.
- If you must balance mechanical strength with corrosion performance: Recognize that the heat treatment for maximum hardness may not provide the best corrosion resistance, and a subsequent tempering step is often a necessary compromise.
- If you are concerned about Stress Corrosion Cracking: Implement a stress-relief anneal after forming or welding to reduce the residual stresses that act as driving forces for failure.
Ultimately, treating heat treatment as a precise instrument for microstructural control is the key to unlocking a material's full corrosion-resistant potential.
Summary Table:
| Heat Treatment Process | Primary Effect on Corrosion Resistance | Key Mechanism |
|---|---|---|
| Annealing / HIP | Enhances | Creates a homogeneous microstructure, eliminating internal galvanic cells. |
| Hot Isostatic Pressing (HIP) | Significantly Enhances | Closes internal pores and voids, preventing crevice corrosion initiation. |
| Stress-Relief Anneal | Enhances | Reduces residual stresses, mitigating Stress Corrosion Cracking (SCC). |
| Sensitizing Cycle (450-850°C) | Severely Degrades | Causes chromium carbide precipitation, creating chromium-depleted zones in stainless steels. |
| Quenching (without tempering) | Can Degrade | Creates hard, electrochemically active phases like martensite, reducing corrosion resistance. |
Optimize Your Material's Performance with KINTEK
Navigating the complex relationship between heat treatment and corrosion resistance requires expert knowledge and the right equipment. The wrong thermal cycle can lead to premature failure, while the correct one can significantly extend your component's service life.
KINTEK specializes in precision lab equipment and consumables that empower you to:
- Achieve Homogeneity: Utilize furnaces for precise annealing to create uniform microstructures.
- Eliminate Defects: Explore Hot Isostatic Pressing (HIP) solutions to produce fully dense, pore-free materials.
- Prevent Sensitization: Accurately control time-temperature profiles to protect stainless steels.
- Relieve Stress: Implement stress-relief cycles to safeguard against Stress Corrosion Cracking.
Our team understands the critical balance between mechanical properties and corrosion performance. Let us help you select the right equipment and processes to fortify your materials against their operating environment.
Contact us today to discuss your specific application and ensure your heat treatment protocols enhance, not hinder, your corrosion resistance goals.
Visual Guide
Related Products
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- Vertical Laboratory Tube Furnace
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
People Also Ask
- What are the advantages of using a vacuum hot pressing furnace over HIP? Optimize Fiber-Foil Composite Production
- Why is the vacuum system of a Vacuum Hot Pressing furnace critical for ODS ferritic stainless steel performance?
- How does the mechanical pressure from a vacuum hot-pressing furnace facilitate the densification of B4C/Al composites?
- How does the degassing stage in a vacuum hot press (VHP) optimize diamond/aluminum composite performance?
- What role does a vacuum hot pressing sintering furnace play in the fabrication of CuCrFeMnNi alloys? Achieve High Purity



















