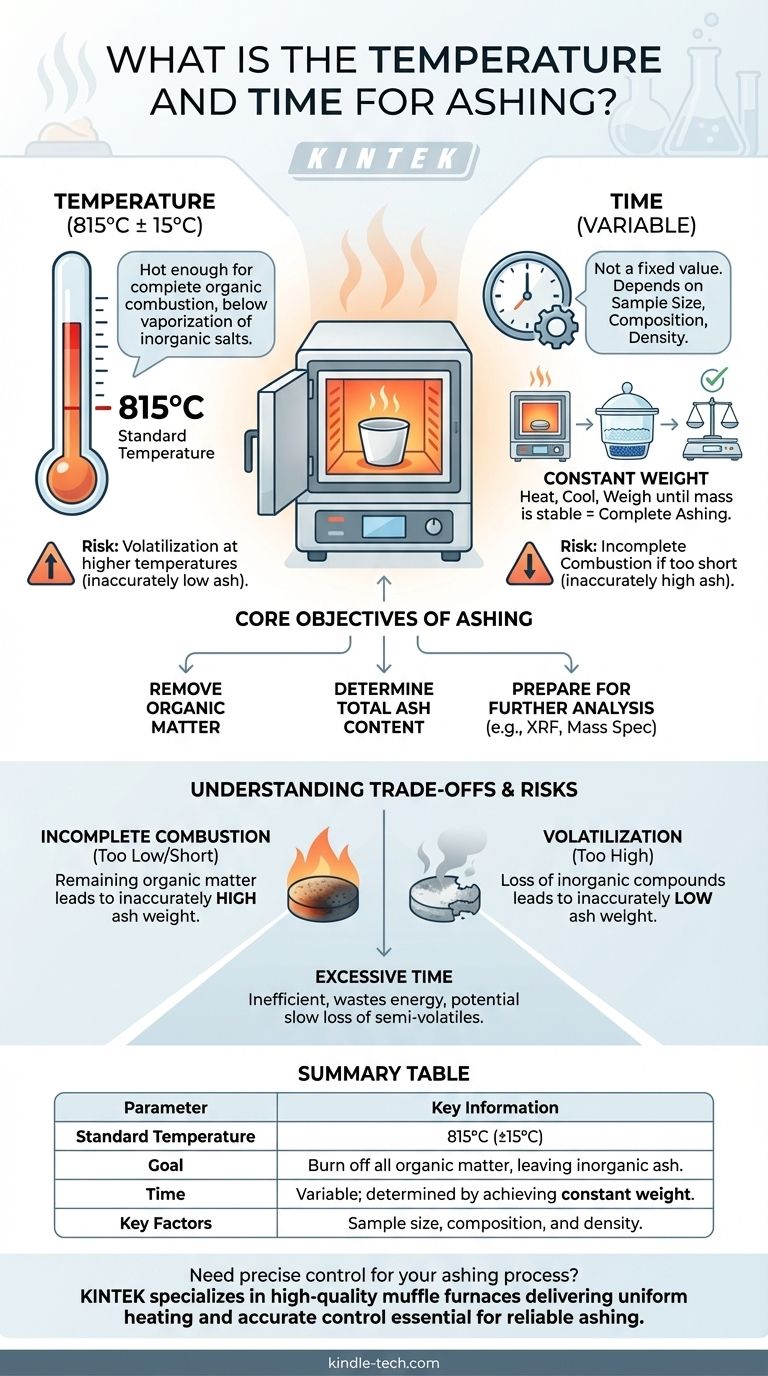
The standard temperature for ashing is typically specified at 815°C with a tolerance of ±15°C. The required time, however, is not a fixed value. It is the duration needed to ensure the sample is completely ashed, meaning all organic material has been burned away.
The objective of ashing is to achieve complete combustion of organic matter, leaving only inorganic residue. Therefore, the correct time and temperature are not universal constants but are the specific conditions required to achieve this goal without altering the inorganic components you intend to measure.

The Core Objective of Ashing
To correctly determine the time and temperature, you must first understand the purpose of your procedure. The process is designed to isolate the inorganic, non-combustible part of a sample.
Removing All Organic Matter
The fundamental goal is to use high heat in the presence of oxygen to burn off all carbon-based organic material, such as proteins, fats, and carbohydrates. What remains is the ash.
Determining Total Ash Content
One of the most common applications is gravimetric analysis, where you measure the total mass of the residual ash. This value represents the total mineral content of the original sample.
Preparing for Further Analysis
Often, the ash itself is the material of interest. It is collected and prepared for subsequent tests like X-ray fluorescence (XRF) or mass spectroscopy to characterize its specific inorganic components.
Key Factors Influencing Time and Temperature
The standard temperature is a reliable starting point, but achieving complete ashing requires careful consideration of several variables.
The Role of Temperature (815°C)
The specified temperature of 815±15°C is a critical balance. It is hot enough to ensure the thorough and efficient combustion of organic compounds.
However, it is generally kept below temperatures where certain inorganic salts might begin to vaporize or decompose, which would result in an inaccurate, artificially low measurement.
Why Time is Variable
There is no universal time for ashing because the duration depends entirely on the sample. Key factors include the sample's size, its composition, and its density.
A large, dense sample with a high organic content will naturally require a longer time in the furnace than a small, porous sample.
The Concept of "Constant Weight"
The only reliable way to know if ashing is complete is to heat the sample until it reaches a constant weight.
The procedure involves heating for a set period, cooling the sample in a desiccator to prevent moisture absorption, and weighing it. This process is repeated until two consecutive weighings show no change, indicating all combustible material is gone.
Understanding the Trade-offs and Risks
Applying the wrong parameters can compromise your results. Understanding the potential pitfalls is essential for accuracy.
Risk of Incomplete Combustion
If the temperature is too low or the time is too short, traces of organic material may remain. This will lead to an inaccurately high ash weight.
Risk of Volatilization
If the temperature is too high, you risk the loss of volatile inorganic compounds, such as chlorides and sulfates. This leads to an inaccurately low ash weight and compromises any further elemental analysis.
The Problem with Excessive Time
While ensuring completeness is vital, arbitrarily prolonging the ashing time is inefficient. It consumes unnecessary energy and, for certain materials, may still lead to the slow loss of semi-volatile components, even at the correct temperature.
How to Determine the Right Parameters for Your Sample
Your specific analytical goal should guide your methodology.
- If your primary focus is determining total ash content: Your objective is to heat the sample until it reaches a constant weight, confirming all organic matter has been eliminated.
- If your primary focus is preparing samples for elemental analysis: You must strictly adhere to the validated method's temperature (e.g., 815°C) to prevent the loss of the specific inorganic elements you intend to measure.
Ultimately, successful ashing depends on understanding your specific sample and analytical goal, not just on following a fixed number.
Summary Table:
| Parameter | Key Information |
|---|---|
| Standard Temperature | 815°C (±15°C) |
| Goal | Burn off all organic matter, leaving inorganic ash. |
| Time | Variable; determined by achieving constant weight. |
| Key Factors | Sample size, composition, and density. |
Need precise control for your ashing process? KINTEK specializes in high-quality laboratory muffle furnaces that deliver the uniform heating and accurate temperature control (like 815°C) essential for reliable ashing and sample preparation. Our equipment helps your lab avoid incomplete combustion or volatilization, ensuring the integrity of your results for gravimetric analysis or subsequent elemental testing. Contact us today to find the perfect furnace for your laboratory's needs!
Contact our experts for a consultation
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the principle of muffle furnace in laboratory? Master Precise High-Temp Heating
- What is the inside material of a muffle furnace? Choose the Right Lining for Your Application
- How to cool a muffle furnace? Ensure Safety and Maximize Equipment Lifespan
- What is the burning temperature of a furnace? From 200°C to 3000°C, It Depends on Your Needs
- What are the working principles of furnace? A Guide to Combustion, Resistance, and Induction Heating



















