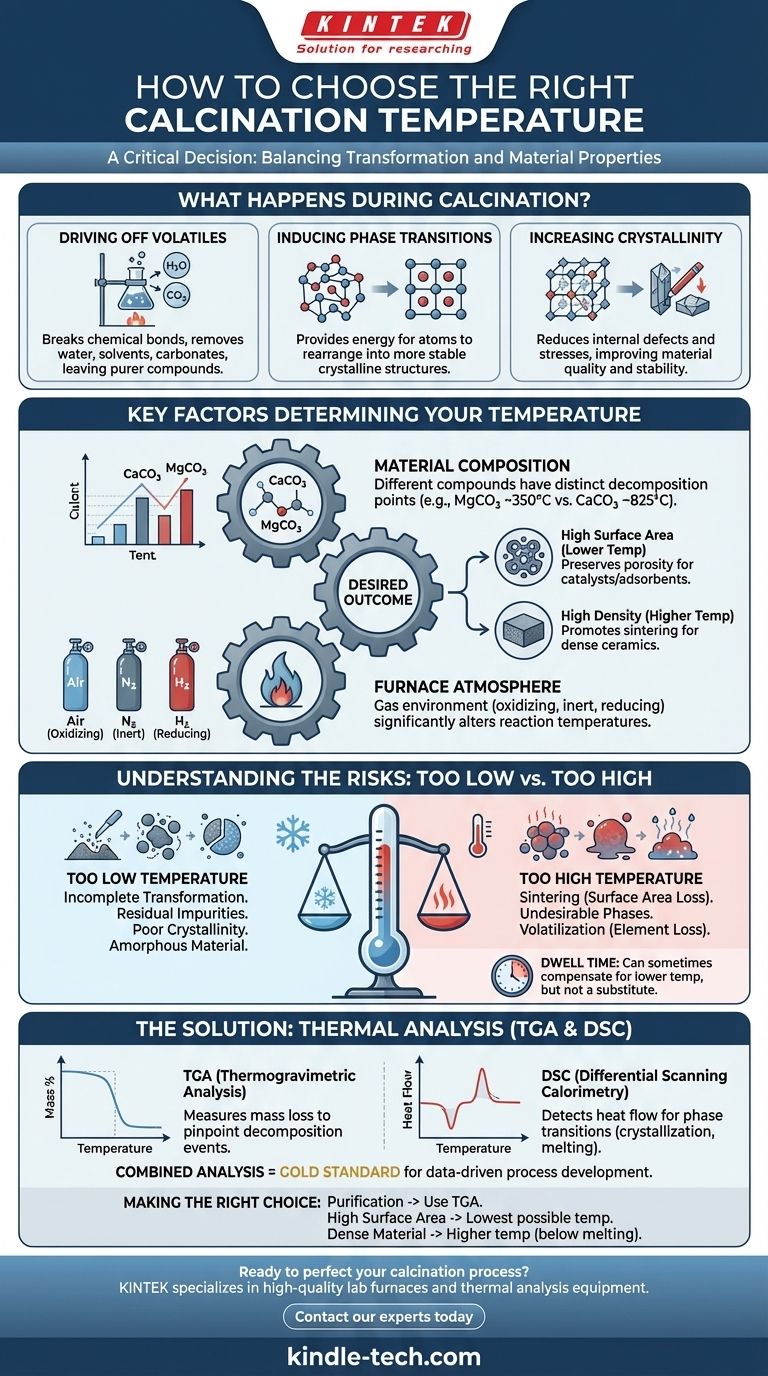
Choosing the right calcination temperature is a critical decision in materials processing, as it directly dictates the final properties of your material. There is no single universal temperature; the ideal value is determined by the specific chemical decomposition, phase transition, or purification you aim to achieve. While many industrial processes operate between 800°C and 1300°C, the correct temperature for your specific application is a careful balance between driving the desired transformation and preventing unwanted effects like particle growth or decomposition.
The ideal calcination temperature is the minimum temperature required to achieve your desired physical or chemical change within a practical timeframe. Exceeding this temperature often introduces negative consequences, such as loss of surface area or the formation of undesirable phases.

What Happens During Calcination?
To choose a temperature, you must first understand what you are trying to accomplish. Calcination is a thermal treatment process used to induce a change in a material's chemical or physical structure through controlled heating in a specific atmosphere.
Driving Off Volatiles
Many precursor materials contain water (both free and bound), solvents from synthesis, or volatile components like carbonates and hydroxides. A primary goal of calcination is to heat the material sufficiently to break these chemical bonds and drive off the resulting gases, leaving behind a purer, more stable compound.
Inducing Phase Transitions
Temperature provides the energy needed for a material's atoms to rearrange themselves. This is often used to convert an amorphous (disordered) material into a crystalline (ordered) one or to transform a material from one crystalline structure (polymorph) to another that is more stable at higher temperatures.
Increasing Crystallinity and Removing Defects
Even if a material is already in the correct crystalline phase, it may contain internal stresses or defects from its initial synthesis. Holding it at an elevated temperature, a process known as annealing, allows atoms to migrate to more stable positions in the crystal lattice. This reduces defects and increases the overall quality and stability of the material.
Key Factors That Determine Your Temperature
Your choice of temperature is not made in a vacuum. It is a function of the material itself, your end goal, and the processing environment.
The Material's Chemical Composition
Different chemical compounds have different thermal stability. For example, calcium carbonate (CaCO₃) begins to decompose into calcium oxide (CaO) and carbon dioxide (CO₂) around 825°C. In contrast, magnesium carbonate (MgCO₃) decomposes at a much lower temperature, starting around 350°C. You must know the thermal properties of your specific precursor.
Your Desired Outcome
The end goal is the most important factor.
- High Surface Area: If you are making a catalyst or adsorbent, you want to preserve a high surface area. This requires using the lowest possible temperature that completes the decomposition, as higher temperatures will cause particles to fuse together (sinter), destroying porosity.
- High Density: If you are preparing a powder for making a dense ceramic, a higher calcination temperature can be beneficial. It creates less reactive, more easily handled powders and can be considered the first step of the sintering process.
The Role of Atmosphere
The gas environment inside the furnace is critical. Calcining in air (an oxidizing atmosphere) is different from calcining in nitrogen (an inert atmosphere) or hydrogen (a reducing atmosphere). The atmosphere can change the temperature at which reactions occur and prevent or promote certain chemical changes.
Understanding the Trade-offs: Too High vs. Too Low
Choosing a temperature is an optimization process. Deviating from the ideal range in either direction will compromise your results.
The Problem with Too Low a Temperature
If the temperature is insufficient, the transformation will be incomplete. This can leave you with residual impurities, an amorphous or mixed-phase material, and poor crystallinity. The material simply has not received enough energy to complete its change.
The Dangers of Too High a Temperature
Excessive heat is often more damaging than insufficient heat.
- Sintering: This is the most common issue. Particles begin to fuse, leading to a dramatic reduction in surface area and reactivity.
- Undesirable Phase Changes: Heating a material too much can cause it to "overshoot" the desired crystal structure and transform into a different, unwanted phase or even melt.
- Volatilization: In multi-component materials, an excessively high temperature can cause one of the more volatile elements (like lead, zinc, or bismuth) to evaporate from the sample, altering its final composition.
The Influence of Dwell Time
Temperature and time are interconnected. A reaction that is sluggish at a lower temperature can sometimes be completed by holding the material at that temperature for a longer period (a longer "dwell time"). However, this cannot compensate for a temperature that is fundamentally too low to initiate the required reaction.
A Practical Method: Thermal Analysis
Instead of guessing, the most reliable way to determine the ideal calcination temperature is through empirical measurement using thermal analysis techniques.
Using Thermogravimetric Analysis (TGA)
TGA measures a material's mass as a function of temperature. By heating a small sample and tracking its weight, you can pinpoint the exact temperatures at which volatile components are driven off. Each sharp drop in the TGA curve represents a decomposition event and gives you a clear target for your calcination temperature.
Using Differential Scanning Calorimetry (DSC)
DSC measures the heat flow into or out of a sample as it is heated. It is exceptionally good at detecting phase transitions that do not involve a change in mass, such as crystallization or melting. An exothermic (heat-releasing) peak on a DSC curve often indicates crystallization, providing a target temperature for achieving that structure.
Combining TGA/DSC for a Complete Picture
Modern analytical instruments often perform TGA and DSC simultaneously. This provides a complete thermal "fingerprint" of your material, showing you both mass-loss events and energetic phase transitions on a single graph. This is the gold standard for developing a robust calcination process.
Making the Right Choice for Your Goal
Use this framework to guide your decision-making process.
- If your primary focus is purification and decomposition: Use thermal analysis (TGA) to identify the temperature at which mass loss is complete, then set your calcination temperature slightly above that point (e.g., 25-50°C higher) to ensure a complete reaction.
- If your primary focus is maximizing surface area: Use the lowest possible temperature that achieves the desired phase and purity. This minimizes sintering and preserves the fine-particle nature of your material.
- If your primary focus is creating a dense, crystalline material: You can use a higher temperature to promote grain growth and defect removal, but be sure to stay safely below any secondary decomposition or melting points identified by DSC.
- If you are unsure: Always begin by performing thermal analysis (TGA/DSC) on your precursor material. The data from this analysis will provide a clear, evidence-based starting point.
Ultimately, a methodical, data-driven approach transforms calcination from a guess into a precisely controlled engineering process.
Summary Table:
| Factor | Impact on Temperature Choice |
|---|---|
| Material Composition | Determines decomposition points (e.g., CaCO₃ at ~825°C). |
| Desired Outcome | High surface area (lower temp) vs. high density (higher temp). |
| Furnace Atmosphere | Oxidizing, inert, or reducing environments alter reaction temps. |
| Dwell Time | Longer times can compensate for slightly lower temperatures. |
| Thermal Analysis (TGA/DSC) | Provides empirical data for precise temperature targeting. |
Ready to perfect your calcination process? The right lab furnace is critical for achieving precise temperature control and consistent results. KINTEK specializes in high-quality lab furnaces and thermal analysis equipment, helping laboratories like yours optimize material synthesis and processing.
Contact our experts today to discuss your specific application and find the ideal solution for your calcination needs.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the capacity of a muffle furnace? Find the Right Size for Your Lab Needs
- What does 'sintered' mean and why is it important to understand? Unlock Advanced Materials & Manufacturing
- How hot does a furnace get in Celsius? From 1100°C to 1800°C for Your Lab Needs
- What is the meaning of debinding? Master the Critical Step to High-Performance Parts
- How does heat affect material strength? Understanding Thermal Degradation and Creep Failure



















