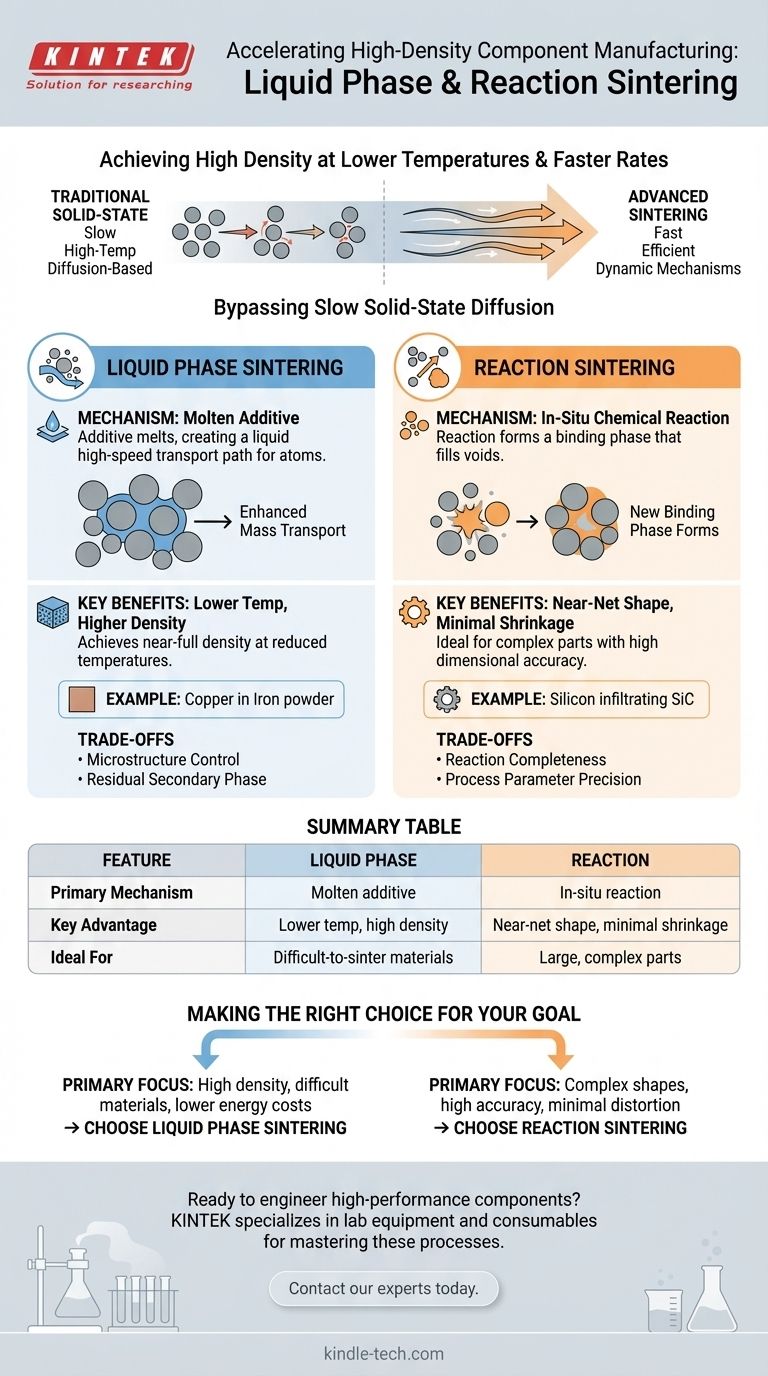
The most attractive feature of both liquid phase and reaction sintering is their ability to achieve high material density at significantly lower temperatures and faster rates than traditional solid-state methods. Liquid phase sintering accomplishes this by using a molten additive to accelerate mass transport, while reaction sintering uses a chemical reaction to form a binding phase that fills pores with minimal shrinkage.
At their core, both methods solve a fundamental manufacturing challenge: how to create strong, dense parts efficiently. They bypass the slow, energy-intensive process of solid-state diffusion by introducing a more dynamic mechanism—a liquid lubricant or an in-situ chemical reaction—to consolidate the material.

The Core Advantage: Bypassing Solid-State Diffusion
Why Standard Sintering is Slow
In traditional solid-state sintering, individual powder particles are bonded together purely through heat and pressure. This process relies on solid-state diffusion, where atoms slowly migrate across particle boundaries.
Achieving full density this way requires extremely high temperatures and long processing times, making it inefficient and costly for many materials.
How a Liquid Phase Accelerates the Process
Liquid phase sintering introduces a small amount of an additive that melts at the sintering temperature, creating a liquid that coats the solid particles. This liquid acts as a high-speed transport path, allowing atoms to move much more freely and rapidly than they could in a solid.
This enhanced mass transport is the central advantage, dramatically speeding up densification and allowing the process to occur at a lower temperature.
How a Chemical Reaction Builds Density
Reaction sintering involves a chemical reaction within the powder compact during heating. This reaction forms a new ceramic or metallic compound that fills the voids between the initial particles.
Because the final volume is largely determined by the reaction product filling existing pores, the component experiences very little to no shrinkage.
A Closer Look at Liquid Phase Sintering
The Mechanism: An Additive That Melts
The process begins by mixing a primary powder with a small amount of a sintering aid (additive). As the material is heated, the additive melts first.
This liquid phase wets the solid particles, filling pores and pulling the grains together into a more dense arrangement through capillary action. A common example is mixing copper powder with iron powder, where the copper melts and fuses the iron particles together.
Key Benefits: Lower Temperature and Higher Density
The primary benefits are a significantly lower sintering temperature and the ability to achieve near-full density. This makes the process more energy-efficient and cost-effective.
It is particularly valuable for materials that are inherently difficult to sinter via solid-state methods, such as certain ceramics and refractory metals.
A Closer Look at Reaction Sintering
The Mechanism: In-Situ Material Formation
In reaction sintering, the initial powder mix contains reactants that will form a binding phase when heated. For example, in reaction-bonded silicon carbide, a porous silicon carbide preform is infiltrated by molten silicon.
The silicon reacts with free carbon in the preform to create new silicon carbide, which bonds the original particles together.
Key Benefits: Near-Net Shape and Low Cost
The standout feature of reaction sintering is extremely small shrinkage. This makes it ideal for producing large or complex-shaped structures with high dimensional accuracy, as the part requires minimal finishing.
Combined with its low sintering temperatures and low production cost, it is a highly attractive manufacturing route for specific applications.
Common Applications
The unique properties of reaction-sintered materials make them suitable for demanding environments. Reaction-sintered silicon carbide, for instance, is frequently used for high-temperature kiln components, rocket nozzles, heat exchangers, and precision optical mirrors.
Understanding the Trade-offs
Liquid Phase Sintering: Microstructure Control is Key
The main challenge in liquid phase sintering is controlling the final microstructure. If not managed properly, the process can lead to excessive grain growth, which can weaken the material.
Furthermore, the liquid phase solidifies upon cooling and remains as a secondary phase in the final part. The properties of this secondary phase can significantly impact the overall performance of the component, and it must be carefully selected.
Reaction Sintering: Reaction Completeness is Critical
The success of reaction sintering hinges on the chemical reaction proceeding to completion. Any unreacted residual material can create weak points and degrade the mechanical or chemical properties of the final product.
Process parameters like temperature, time, and atmosphere must be precisely controlled to ensure a complete and uniform reaction throughout the part.
Making the Right Choice for Your Goal
By understanding the distinct advantages of each process, you can align your manufacturing strategy with your specific engineering goal.
- If your primary focus is achieving high density in a difficult-to-sinter material while lowering energy costs: Liquid phase sintering is an excellent choice due to its enhanced mass transport mechanism.
- If your primary focus is manufacturing large, complex parts with high dimensional accuracy and minimal distortion: Reaction sintering's near-zero shrinkage makes it a uniquely powerful and cost-effective solution.
Ultimately, selecting the correct advanced sintering method transforms a powdered material into a high-performance component engineered for its specific purpose.
Summary Table:
| Feature | Liquid Phase Sintering | Reaction Sintering |
|---|---|---|
| Primary Mechanism | Molten additive enhances mass transport | In-situ chemical reaction forms binding phase |
| Key Advantage | Lower temperature, high density | Near-net shape, minimal shrinkage |
| Ideal For | Difficult-to-sinter materials (e.g., ceramics, refractory metals) | Large, complex parts requiring high dimensional accuracy |
| Main Consideration | Microstructure and secondary phase control | Completeness of the chemical reaction |
Ready to engineer high-performance components with advanced sintering?
At KINTEK, we specialize in providing the lab equipment and consumables needed to master liquid phase and reaction sintering processes. Whether you are developing new materials or optimizing production for complex parts, our solutions help you achieve superior density, precise dimensional control, and significant energy savings.
Contact our experts today to discuss how we can support your specific laboratory and manufacturing goals.
Visual Guide
Related Products
- Vacuum Hot Press Furnace Machine Heated Vacuum Press
- Vacuum Hot Press Furnace Heated Vacuum Press Machine Tube Furnace
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Automatic High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
People Also Ask
- How does the uniaxial pressing function of a vacuum hot press furnace influence the microstructure of ZrC-SiC ceramics?
- What role does a high-temperature hot press play in the sintering of NITE-SiC? Optimize Your Densification Process
- What conditions does a Vacuum Hot Pressing Furnace provide for Copper-MoS2-Mo composites? Achieve Peak Densification
- Why is a vacuum essential for sintering metal-ceramic composites? Achieve Pure, High-Density Results
- What are the advantages of using a vacuum hot press for CuCr50? Achieve Superior Density & Purity in Alloy Production



















