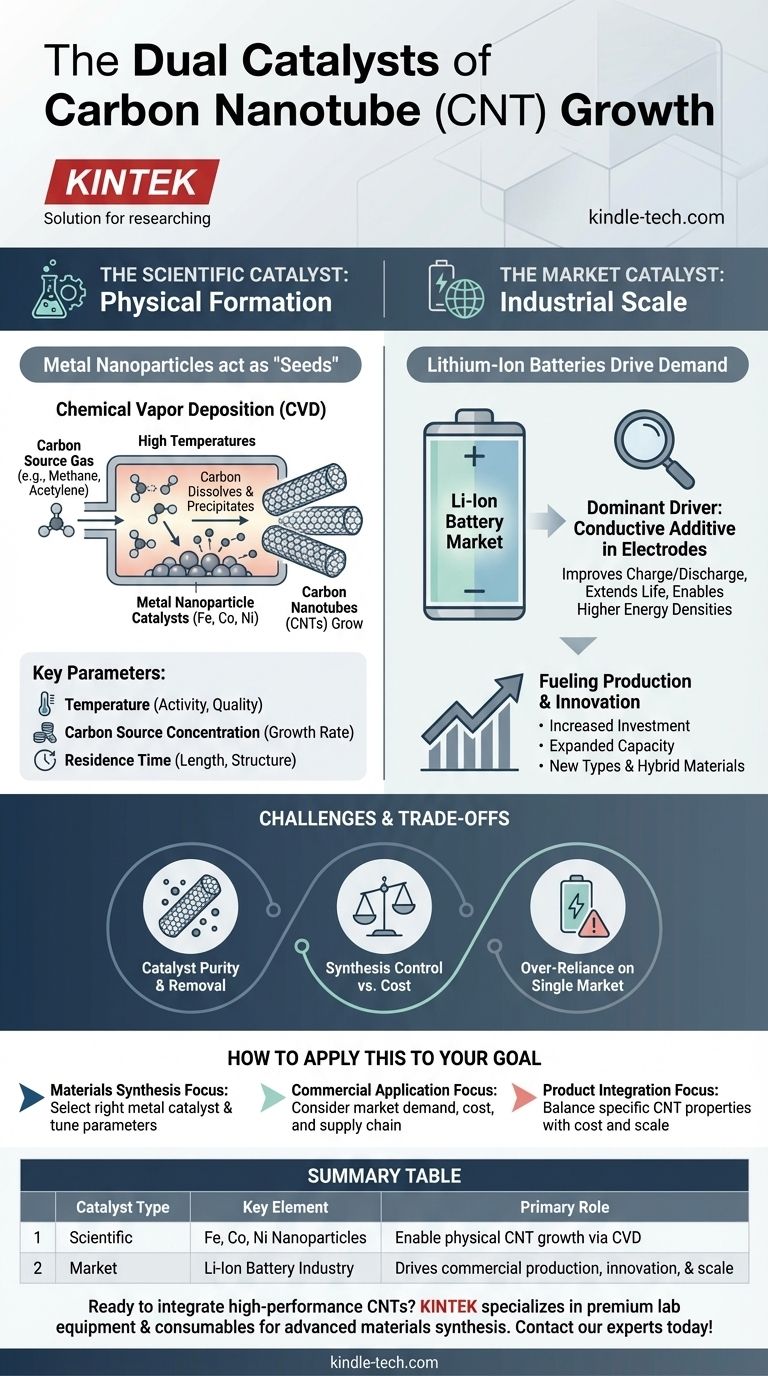
In short, the catalysts used to physically grow carbon nanotubes (CNTs) are typically nanoparticles of transition metals, most commonly iron, cobalt, or nickel. These metal particles act as the "seeds" from which the carbon tube structure precipitates and grows during synthesis processes like chemical vapor deposition (CVD).
Understanding the "catalyst" for carbon nanotubes requires looking at two distinct forces: the scientific catalyst (metal nanoparticles) that enables their physical formation, and the market catalyst (lithium-ion batteries) that drives their commercial production and innovation.

The Scientific Catalyst: How Nanotubes Physically Form
The physical growth of a carbon nanotube from a carbon source gas (like methane or acetylene) is not a spontaneous process. It requires a specific catalytic surface to initiate and sustain the reaction.
The Role of Metal Nanoparticles
The most effective and widely used catalysts are iron (Fe), cobalt (Co), and nickel (Ni). These metals, when prepared as nanoparticles, have a unique ability at high temperatures to break down carbon-containing molecules.
Carbon atoms dissolve into the surface of the metal nanoparticle. When the metal becomes supersaturated with carbon, the carbon atoms precipitate out in a stable, cylindrical honeycomb lattice, forming the wall of the nanotube.
The Importance of Synthesis Parameters
While the metal catalyst is the seed, the final properties of the CNTs are governed by the environment. Key operating parameters include:
- Temperature: Determines the catalytic activity and the quality of the resulting nanotubes.
- Carbon Source Concentration: The amount of available carbon feedstock influences the growth rate.
- Residence Time: The duration the carbon source is exposed to the catalyst affects the length and structure of the CNTs.
The Market Catalyst: Driving Industrial Scale
Beyond the lab, the explosive growth of the CNT industry is being catalyzed by overwhelming demand from a specific sector. This economic driver is just as critical to the availability and advancement of CNTs as the metal particles are to their physical creation.
The Dominance of Lithium-Ion Batteries
The single largest driver for CNT production today is the lithium-ion battery market. CNTs are used as a highly effective conductive additive in battery electrodes (both anodes and cathodes).
Their high aspect ratio and exceptional electrical conductivity create a robust conductive network within the electrode, improving charge/discharge rates, extending battery life, and enabling higher energy densities.
Fueling Production and Innovation
This booming demand from the energy storage sector is fueling massive investment. Production volumes are increasing, and major players are expanding their capacity to meet this need.
This market pull also drives technical innovation, pushing research into new types of CNTs, hybrid materials, and advanced products like highly conductive continuous yarns for next-generation applications.
Understanding the Trade-offs
While powerful, both the scientific and market catalysts present distinct challenges that are critical to understand.
Catalyst Purity and Removal
A significant challenge in CNT manufacturing is that the metal catalyst particles used for growth remain as an impurity in the final product. For high-performance applications, especially in electronics and medicine, these metallic residues must be removed through complex and often costly purification steps.
Synthesis Control vs. Cost
The synthesis parameters required to produce high-quality, uniform CNTs (e.g., single-chirality tubes) are difficult and expensive to maintain at an industrial scale. This creates a constant trade-off between producing premium, specialized CNTs and low-cost, bulk-grade material.
Over-Reliance on a Single Market
The CNT industry's heavy dependence on the lithium-ion battery market is both a strength and a potential risk. Any major shift in battery chemistry or a slowdown in the electric vehicle sector could significantly impact the entire CNT market.
How to Apply This to Your Goal
Your perspective on the "catalyst" for CNTs depends entirely on your objective.
- If your primary focus is materials synthesis: Your attention should be on selecting the right metal catalyst (Fe, Co, Ni) and tuning synthesis parameters to control the physical properties of the nanotubes.
- If your primary focus is commercial application: Your concern is the market catalyst, where the demand from the battery industry dictates cost, availability, and the supply chain for bulk CNTs.
- If your primary focus is product integration: You must consider both, balancing the need for specific CNT properties (determined by synthesis) with the cost and scale offered by market drivers.
Ultimately, grasping both the scientific mechanism and the economic forces provides a complete picture of the world of carbon nanotubes.
Summary Table:
| Catalyst Type | Key Element | Primary Role |
|---|---|---|
| Scientific | Iron, Cobalt, Nickel Nanoparticles | Enable physical CNT growth via Chemical Vapor Deposition (CVD) |
| Market | Lithium-Ion Battery Industry | Drives commercial production, innovation, and scale |
Ready to integrate high-performance carbon nanotubes into your research or product development? KINTEK specializes in providing premium lab equipment and consumables tailored for advanced materials synthesis, including CNT production. Our expertise ensures you have the right tools to optimize catalyst selection and synthesis parameters for your specific application. Contact our experts today to discuss how we can support your laboratory's innovation goals!
Visual Guide
Related Products
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical Laboratory Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
People Also Ask
- What do optical coatings do? Control Light for Superior Optical Performance
- What are pure silicon sputtering targets? Precision Source for High-Performance Thin Films
- What is the effect of temperature on graphene oxide? Master Thermal Reduction for Precise Material Properties
- What are the advantages of thin film substrate? Enhance Surface Functionality for Superior Products
- What nanomaterials are synthesized by chemical vapor deposition? Building High-Performance Materials with Precision
- What is the alternative material for graphene? Discover the Top 2D Materials for Your Specific Application
- What is the sputtering process in semiconductors? A Guide to Precision Thin Film Deposition
- What materials and techniques are used to create thin films? Master Precision Deposition for Advanced Lab Research



















