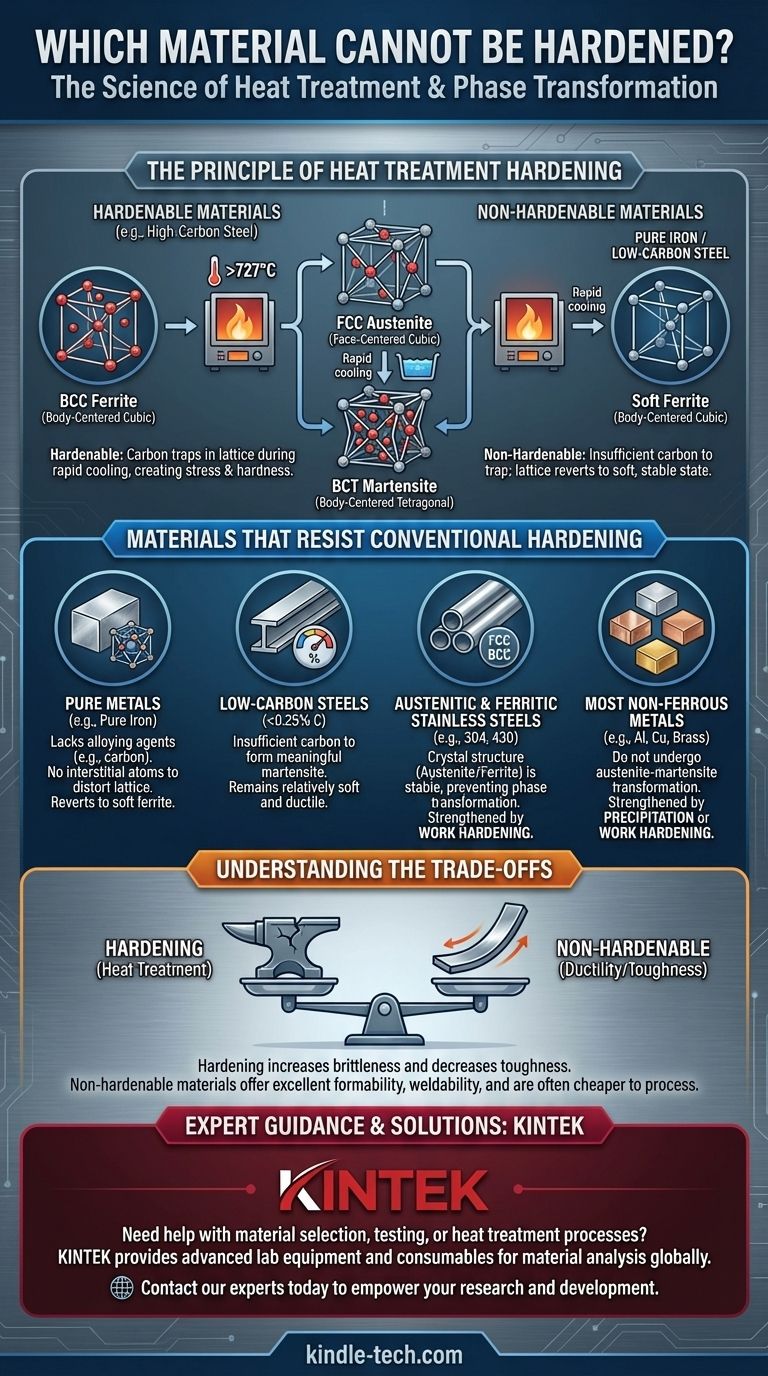
Fundamentally, materials that cannot be hardened by conventional heat treatment are those that lack the specific internal crystal structure and chemical composition needed to undergo a phase transformation. This includes pure metals like iron, most non-ferrous alloys like aluminum and copper in their pure state, low-carbon steels, and specific families of stainless steel such as the austenitic and ferritic grades. These materials either lack the necessary alloying elements (like carbon) or have a crystal structure that remains stable when heated and cooled.
The ability to harden a metal is not an inherent property but a consequence of its internal structure. True hardening via heat treatment relies on an alloy's ability to transform its crystal lattice into a highly stressed, distorted state—a change that many common and useful metals are simply incapable of making.

The Principle of Heat Treatment Hardening
To understand what cannot be hardened, we must first understand what hardening is. The most common method, quench hardening, is specific to certain steels and a few other alloys.
The Role of Carbon and Phase Transformation
The classic example is steel. When a medium or high-carbon steel is heated above a critical temperature (around 727°C or 1340°F), its crystal structure changes from a Body-Centered Cubic (BCC) arrangement, called ferrite, into a Face-Centered Cubic (FCC) structure called austenite.
Austenite has a unique ability to dissolve a significant amount of carbon atoms within its lattice.
The Quench and the Martensite Trap
If this steel is then cooled very rapidly (quenched), the carbon atoms do not have time to escape. The iron lattice is forced to snap back towards its BCC structure, but the trapped carbon atoms distort it into a new, highly strained Body-Centered Tetragonal (BCT) structure called martensite.
This internal stress and distortion are what make the steel exceptionally hard and brittle. Without this specific transformation, this type of hardening is impossible.
Materials That Resist Heat Treatment Hardening
Based on the principle above, we can identify several categories of materials that cannot be hardened by quenching.
Pure Metals (e.g., Pure Iron)
Pure iron, without a sufficient amount of a hardening agent like carbon, cannot be significantly hardened by heat treatment. While it undergoes the same ferrite-to-austenite phase change when heated, there are no interstitial atoms to trap and distort the lattice upon cooling. The structure simply reverts to soft ferrite.
Low-Carbon Steels
This is one of the most common "non-hardenable" materials. Steels with a carbon content below approximately 0.25% do not have enough dissolved carbon to produce a meaningful amount of martensite upon quenching. The resulting material remains relatively soft and ductile, which is why these steels are prized for their formability and weldability.
Austenitic Stainless Steels (e.g., 304, 316)
This family of stainless steels has a chemical composition (high in nickel and chromium) that keeps its crystal structure in the FCC austenite phase even at room temperature. Since it doesn't transform out of the austenite phase upon cooling, the martensitic transformation cannot occur.
It's critical to note that these steels can be hardened, but through a completely different mechanism called work hardening (or strain hardening), which involves physically deforming the metal at room temperature.
Ferritic Stainless Steels (e.g., 430)
Similar to austenitic grades, ferritic stainless steels have a crystal structure—in this case, BCC ferrite—that is stable at all temperatures up to its melting point. With no phase change, there is no opportunity for quench hardening.
Most Non-Ferrous Metals
Metals like aluminum, copper, brass, and titanium do not undergo the austenite-martensite transformation. Their pure forms can only be hardened through work hardening. However, many of their alloys can be hardened by a different method called precipitation hardening (or age hardening), which involves heating to dissolve alloying elements and then aging to form nano-scale precipitates that obstruct dislocation movement, thereby strengthening the material.
Understanding the Trade-offs
Choosing a material involves balancing its properties. The inability to be hardened is not always a disadvantage.
Hardness vs. Toughness and Ductility
The primary trade-off of hardening is a loss of toughness and ductility. A hardened material is more brittle and will fracture under impact rather than bend. Materials that cannot be hardened, like low-carbon steel, retain their excellent ductility, making them easy to form, bend, and weld without cracking.
Work Hardening as an Alternative
For materials like 304 stainless steel or copper, the lack of heat treatability is overcome by work hardening. This process hardens the material as it is being formed (e.g., drawn into a wire or rolled into a sheet). This can be a benefit in manufacturing, as the final product is strengthened by the very process that creates it.
Cost and Complexity
Hardenable steels require precise thermal processing (heating, soaking, quenching, and tempering), which adds significant cost and complexity to manufacturing. Non-hardenable materials are often simpler and cheaper to process, making them the default choice for general structural and fabrication applications where extreme hardness is not required.
Making the Right Choice for Your Goal
Your choice depends entirely on the engineering requirements of your project.
- If your primary focus is extreme hardness and wear resistance: You must select a medium-to-high carbon steel or a specialized tool steel that is designed for heat treatment.
- If your primary focus is corrosion resistance and ductility: An austenitic stainless steel (like 304) is an excellent choice, but you must rely on work hardening for any increase in strength.
- If your primary focus is low cost, formability, and weldability: A low-carbon steel is the ideal material precisely because it cannot be inadvertently hardened and made brittle during welding or forming.
Understanding why a material can or cannot be hardened is the key to selecting the right one for your specific engineering challenge.
Summary Table:
| Material Category | Key Examples | Why It Cannot Be Hardened by Quenching | Alternative Strengthening Method |
|---|---|---|---|
| Pure Metals | Pure Iron | Lacks carbon/alloying elements for phase transformation | Work Hardening |
| Low-Carbon Steels | AISI 1010 | Carbon content too low (<0.25%) to form martensite | Work Hardening |
| Austenitic Stainless Steels | 304, 316 | Stable FCC austenite structure prevents transformation | Work Hardening |
| Ferritic Stainless Steels | 430 | Stable BCC ferrite structure prevents transformation | Work Hardening |
| Most Non-Ferrous Metals | Pure Aluminum, Copper | No austenite-martensite transformation | Precipitation/Work Hardening |
Need Expert Guidance on Material Selection and Heat Treatment?
Choosing the right material is critical to your project's success. Whether you require extreme hardness, corrosion resistance, or superior ductility, KINTEK is here to help. We specialize in providing advanced lab equipment and consumables for material testing and analysis, serving laboratories and research facilities worldwide.
We can help you:
- Accurately test material properties and hardening capabilities
- Select the optimal heat treatment processes for your specific alloys
- Ensure your materials meet precise engineering specifications
Let us empower your research and development with reliable, precision equipment.
Contact our experts today to discuss your material science needs and discover how KINTEK's solutions can enhance your laboratory's capabilities.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Brazing Furnace
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
People Also Ask
- What are the three main heat treatments? Mastering Annealing, Hardening & Tempering
- How does heat treatment process work? Tailor Material Properties for Your Application
- What are the four types of heat treating processes? Master Annealing, Normalizing, Hardening, and Tempering
- What are the parts of a vacuum furnace? A Guide to the 5 Core Systems
- What are the different types of heat treatment process for steel? Tailor Strength, Hardness & Toughness



















