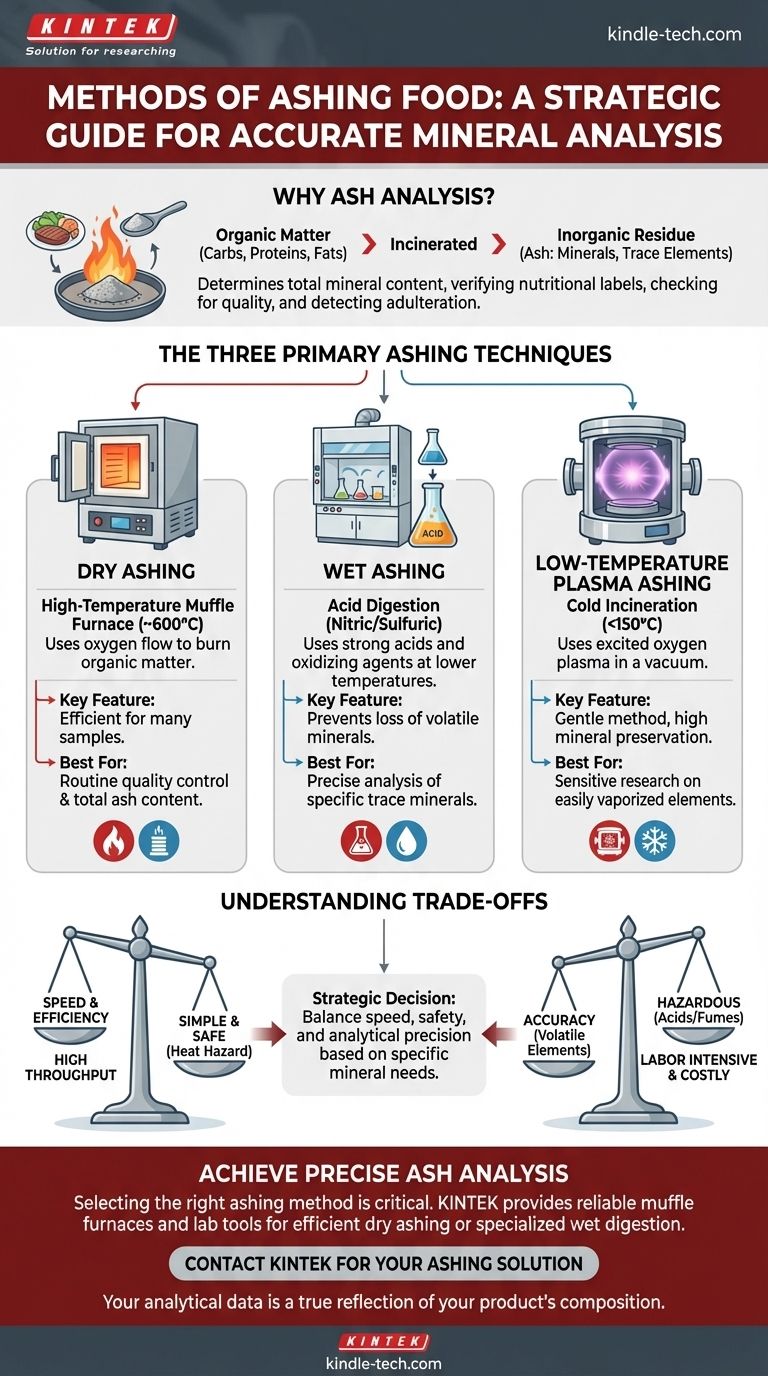
The primary methods for ashing food are dry ashing, wet ashing, and low-temperature ashing. This process involves the high-temperature combustion of a sample to burn off all organic matter, leaving only the inorganic mineral components behind. The choice of method is critical and depends entirely on the analytical goal, sample type, and required specifications.
Choosing the right ashing method is a strategic decision balancing speed, safety, and analytical precision. The core question is not just how to measure ash, but which minerals you need to measure accurately.

The Purpose of Ash Analysis in Food
Before comparing methods, it's crucial to understand why this process is performed. Ashing is a foundational technique in food science for determining the total mineral content of a product.
What "Ash" Represents
Ash is the inorganic residue that remains after all organic compounds—primarily carbohydrates, proteins, and fats—have been completely incinerated. This residue consists of the essential minerals, trace elements, and metallic contaminants present in the original sample.
Why It's a Critical Metric
The ash content is a key indicator of food quality and nutritional value. It is used to verify nutritional labels, check for the presence of certain minerals, and even detect potential adulteration where inorganic fillers might have been added.
A Breakdown of Ashing Techniques
Each ashing method serves a different purpose, with distinct advantages and disadvantages related to temperature, safety, and the preservation of specific minerals.
Dry Ashing
This is the most common method used in the food industry for determining total mineral content. The sample is placed in a high-temperature muffle furnace, typically heated to around 600°C (1112°F). A flow of oxygen helps convert the sample's non-combustible elements into stable oxides and sulfates. The remaining dry weight is the total ash.
Wet Ashing
Wet ashing, or wet digestion, is used when analyzing for specific trace minerals that might be lost at the high temperatures of a muffle furnace. Instead of heat alone, this method uses a combination of strong acids and oxidizing agents (like nitric or sulfuric acid) to digest the organic matter at much lower temperatures.
Low-Temperature Plasma Ashing
This is a more specialized and less common technique. It uses a vacuum chamber where excited oxygen plasma is used to "cold-incinerate" the organic material at temperatures typically below 150°C. This is the most gentle method available.
Understanding the Trade-offs
No single method is universally superior. The correct choice depends on a clear understanding of the trade-offs between speed, accuracy, and cost.
Volatility vs. Speed
The primary trade-off is between the speed of dry ashing and the accuracy of wet ashing for certain elements. The extreme heat of dry ashing can cause volatile minerals (such as mercury, lead, and zinc) to vaporize and escape, leading to an inaccurate, understated result for those specific elements.
Safety and Handling
Dry ashing is relatively simple and safe, with the primary hazard being the high heat of the furnace. In contrast, wet ashing is significantly more hazardous, requiring skilled handling of highly corrosive acids and constant supervision in a fume hood.
Throughput and Cost
For routine quality control, dry ashing is highly efficient. A muffle furnace can process many samples simultaneously with minimal hands-on time. Wet and low-temperature ashing are more labor-intensive and require more expensive equipment, making them less suitable for high-throughput environments.
Making the Right Choice for Your Goal
Your analytical objective should be the definitive guide for selecting an ashing technique.
- If your primary focus is routine quality control or general nutritional labeling for total ash: Dry ashing is the most efficient and practical method.
- If your primary focus is the precise analysis of specific volatile trace minerals: Wet ashing is required to prevent mineral loss and ensure accurate quantification.
- If your primary focus is highly sensitive research on easily vaporized elements: Low-temperature plasma ashing offers the highest degree of mineral preservation, though at a significant cost in time and equipment.
Ultimately, selecting the correct ashing technique ensures your analytical data is not just a number, but a true reflection of the product's mineral composition.
Summary Table:
| Method | Key Feature | Best For |
|---|---|---|
| Dry Ashing | High-temperature muffle furnace (~600°C) | Routine quality control & total ash content |
| Wet Ashing | Acid digestion at lower temperatures | Precise analysis of volatile trace minerals |
| Low-Temperature Plasma Ashing | Cold incineration (<150°C) | Sensitive research on easily vaporized elements |
Achieve precise and reliable ash analysis in your lab. Selecting the right ashing method is critical for accurate mineral quantification. KINTEK specializes in laboratory equipment and consumables, providing the reliable muffle furnaces and lab tools you need for efficient dry ashing or specialized wet digestion. Our experts can help you choose the ideal solution for your specific food testing requirements. Contact our team today to discuss your ashing application and ensure your analytical data is a true reflection of your product's composition.
Visual Guide
Related Products
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is a muffle furnace used for? Achieve Pure, High-Temperature Processing
- How do you keep a sample in a muffle furnace? A Guide to Safe and Accurate Placement
- What is a muffle furnace in the environment? Achieve Clean, Contaminant-Free Heating
- What is the heat capacity of a muffle furnace? Understanding Thermal Mass for Optimal Performance
- What are the four steps to the heat treating process? Master the 3 Core Stages for Superior Results



















